- Who We Are
- Services
- Europe
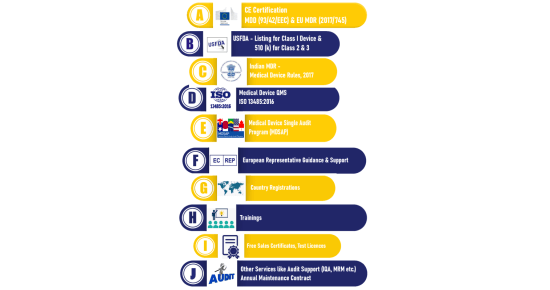
- CE Mark for Medical Device
- CE for In Vitro Diagnostic Device
- Clinical Evaluation
- Post Market Surveillance
- CE approvals for Vacuum Blood Collection Tubes
- CE for Medical Device Software – SaMD
- CE For Personal Protective Equipment
- Gap Analysis
- European Authorized Representative
- Unique Device Identification
- European Database On Medical Devices
- Liability Insurance
- European Free Sale Certificate
- Medical Device (QMS)
- India
- UK
- USA
- Others
- Europe
- Pricing
- RegXpert
- Contact Us