EUDAMED- EUROPEAN DATABASE ON MEDICAL DEVICE
EUDAMED database – European Database on Medical Devices, is the IT system engineered by European Commission to put into effect Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnostic devices.
EUDAMED is multipurpose, acts as a registration and a collaborative, a notification and a dissemination system (open to the public), and be interoperable.
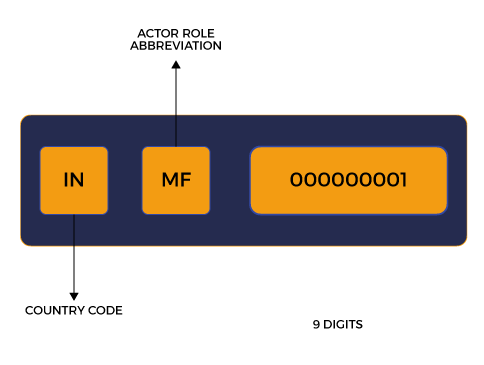
Article 31 demands economic operators such as Manufacturers, Authorised Representatives, and Importers to acquire a Single Registration Number or “SRN” via EUDAMED Registration.
Article 29 calls for manufacturers to add records for every device, such as its UDI data within the EUDAMED Database. EUDAMED database is established around 6 interconnected modules and a public and restricted website:
- Actor Registration Module
- UDI Database and Devices Registration Module
- Notified Bodies and Certificates
- Clinical Investigations and Performance Studies
- Vigilance and Post-Market Surveillance
- Market Surveillance
The Actor Registration Module became active on the 1st of December 2020. It is the first of the six modules.