Validation
Validation is defined as confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been fulfilled. The organization shall validate the processes where the resulting output cannot be verified by subsequent monitoring and the deficiencies become apparent only after the product is in use or the service has been delivered.
Medical device manufacturers are required to have a documented procedure for validation along with well defined responsibilities. Process validation is an important aspect and can be thought of as a separate, independent field.
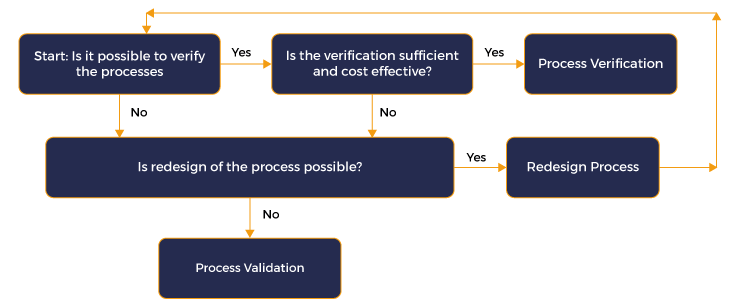
Evaluation of Process for Validation
Before Planning validation, one should evaluate whether the process needs to be validated or verified. Now, what is the difference between a process that needs verification and process that needs validation. A very effective diagram for evaluating whether a process needs to be validated or verified is included below.