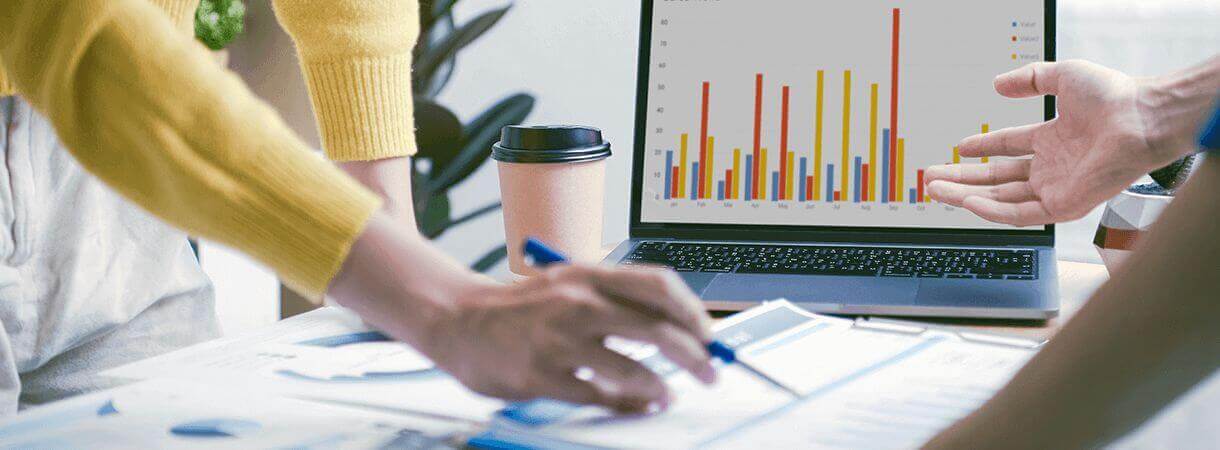
Periodic safety update report
As per EU MDR and IVDR, PMSR (Post-Market Surveillance Report) shall be prepared for Class I medical devices and Class A and B in-vitro diagnostic devices. The report summarizes the results and conclusions of the analyses of the post-market surveillance data gathered as per the post-market surveillance plan. Additionally, periodic safety update reports (PSURs) are required to be generated, providing a comprehensive review of the safety and performance of the device throughout its lifecycle.
Manufacturers of class IIa, class IIb and class III medical devices as well as class C and Class D IVD devices shall prepare a Periodic Safety Update Report (PSUR) by summarizing the results and conclusions of the analyses of the Post-Market Surveillance (PMS) data gathered as a result of the Post-Market Surveillance plan together with a rationale and description of any preventive and corrective actions taken. Periodic Safety Update Report (PSUR) shall be a part of technical documentation for all the categories mentioned above but not for custom-made devices.
Throughout the lifetime of the device, the Periodic Safety Update Report (PSUR) should be updated with the following aspects:
When should one update PSUR?
- Manufacturers of Class IIb and Class III medical devices and Class C and Class D IVD devices shall update the PSUR at least annually.
- Manufacturers of class IIa medical devices shall update the PSUR when necessary and at least every two years.
Who should submit PSURs?
Periodic Safety Update Reports (PSURs) are critical documents that manufacturers of medical devices and in vitro diagnostic (IVD) devices must prepare and submit. PSURs are a regulatory requirement under the European Union Medical Device Regulation (EU MDR) and In Vitro Diagnostic Regulation (IVDR) for all Class IIa, IIb, III, and Class C and D IVD devices.
The responsibility for preparing and submitting Periodic Safety Update Reports (PSURs) lies with the manufacturer or the authorized representative (AR) in the case of non-EU manufacturers. These entities must continuously monitor the safety and performance of their devices in the market. PSURs consolidate post-market surveillance (PMS) data, clinical performance, and risk assessments to ensure the device remains safe and effective throughout its lifecycle.
Additionally, manufacturers must ensure the PSUR is regularly updated and readily available to competent authorities and notified bodies during inspections or audits. This proactive approach not only supports regulatory compliance but also demonstrates the manufacturer’s commitment to patient safety and product quality. Submitting accurate and timely PSURs is essential to maintain market access and uphold trust in medical device performance.
Submission of PSUR
- For class III medical devices or implantable devices, as well as class C and Class D IVD devices manufacturers shall submit a Periodic Safety Update Report (PSUR) by means of the electronic system to the notified body involved in the conformity assessment
- For medical devices other than those mentioned in the above point, manufacturers shall make a Periodic Safety Update Report (PSUR) available to the notified body involved in the conformity assessment and, upon request, to competent authorities.
- The manufacturers must submit a Periodic Safety Update Report in compliance with Annex III – Technical Documentation of Post Market Surveillance.
Role of consultant in writing PSUR
We, Maven Profcon Services LLP will take upon the Post-Market Surveillance activities upon request, including Periodic Safety Update Report and Post Market Surveillance Report!!!
How to write a Periodic Safety Update Report for Medical & IVD devices
Writing a Periodic Safety Update Report (PSUR) for medical devices and in vitro diagnostic (IVD) devices involves compiling and analyzing post-market surveillance data to provide an overview of the safety and performance of the device. While the specific requirements and format may vary based on regulatory guidelines and regional standards, here is a general outline of the steps you can follow to write a PSUR for medical and IVD devices, along with a periodic safety update report template to streamline the process:
Understand Regulatory Requirements:
Familiarize yourself with the specific regulatory requirements for PSURs in your region, whether it’s under the European Union’s Medical Device Regulation (MDR), the In Vitro Diagnostic Regulation (IVDR), or other relevant regulations. Ensure that you have a clear understanding of what needs to be included in the report and the reporting frequency.
Gather Post-Market Surveillance Data:
Collect relevant post-market surveillance data, including information on adverse events, complaints, clinical investigations, field safety corrective actions (recalls), post-market clinical follow-up (PMCF) studies, and any other safety-related data. Ensure that you have comprehensive and up-to-date data for the reporting period.
Analyze Data:
Analyze the collected data to identify trends, patterns, and potential safety concerns. Evaluate the device’s benefit-risk profile based on the available data. Assess the impact of any corrective or preventive actions taken during the reporting period.
Prepare the Report:
Organize the report based on the regulatory requirements and guidelines. Include the following sections:
- Executive Summary: Provide a concise overview of the main findings, conclusions, and actions taken during the reporting period.
- Introduction: Introduce the device and provide context for the report.
- Device Description: Describe the device’s intended use, design, and specifications.
- Post-Market Surveillance Methodology: Explain the methods used to collect and analyze post-market surveillance data.
- Adverse Event Data: Present adverse event data, including a summary of reported events, severity, outcomes, and any changes in reporting trends.
- Benefit-Risk Assessment: Evaluate the device’s benefit-risk balance based on the post-market data, clinical experience, and other available information.
- Field Safety Corrective Actions: Detail any recalls, field safety corrective actions, or other measures taken to address safety issues.
- Post-Market Clinical Follow-Up (PMCF): Include the results of any PMCF studies conducted during the reporting period.
- Conclusions and Recommendations: Summarize the main findings, highlight significant safety concerns, and provide recommendations for any necessary actions or changes.
- References: Cite relevant scientific literature, guidance documents, and regulations that support your analysis.
Review and Approval:
Have the PSUR reviewed by cross-functional teams, including regulatory affairs, quality assurance, clinical affairs, and other relevant departments. Ensure that the report is accurate, comprehensive, and aligned with the regulatory requirements.
Submission:
If required by regulatory authorities, submit the PSUR within the specified timeframe and through the appropriate channels. Follow the submission procedures outlined in the regulations.
Remember, writing a Periodic safety update report (PSUR) requires a thorough understanding of the device, its post-market performance, and the applicable regulatory framework. It’s advisable to work closely with regulatory experts and professionals familiar with post-market surveillance to ensure that your report meets all the necessary requirements and accurately reflects the safety and performance of your medical or IVD device.
For more information on PSUR, see our video
FAQ:
Conclusions and results of Post market surveillance data which includes Complaint data, risk monitoring, vigilance, sales data; main findings of PMCF, conclusions of benefit risk determination, an estimate evaluation of the size and other characteristics of the population using the device and, where practicable, the usage frequency of the device.
PSUR update helps us in determining and analyzing the safety of the medical devices by identifying risks, evaluation benefit-risk ratio. It helps the manufacturer to implement necessary preventive actions.
The frequency of PSUR updates depends on the device classification. For Class IIb and Class III medical devices, and Class C and D IVD devices, PSURs must be updated at least annually. For Class IIa medical devices, updates are required at least every two years or sooner if necessary. Regular updates ensure continuous monitoring of device safety and performance throughout its lifecycle, as required under EU MDR and IVDR.
A Periodic Safety Update Report (PSUR) in India is a regulatory document that provides a summary of the safety and performance of a medical device or IVD device based on post-market surveillance data. While India follows its own Medical Device Rules (MDR), manufacturers exporting to the EU or following international standards may still prepare PSURs to meet global expectations. It includes adverse events, benefit-risk evaluation, and corrective actions taken.
A Periodic Safety Update Report (PSUR) is required to ensure continuous monitoring of a medical or IVD device’s safety and performance after it is placed on the market. It helps identify and assess potential risks, supports benefit-risk evaluation, and documents any corrective actions taken. Regulatory authorities use PSURs to verify ongoing device safety, and manufacturers must regularly update and submit them as part of their post-market surveillance obligations under EU MDR and IVDR.