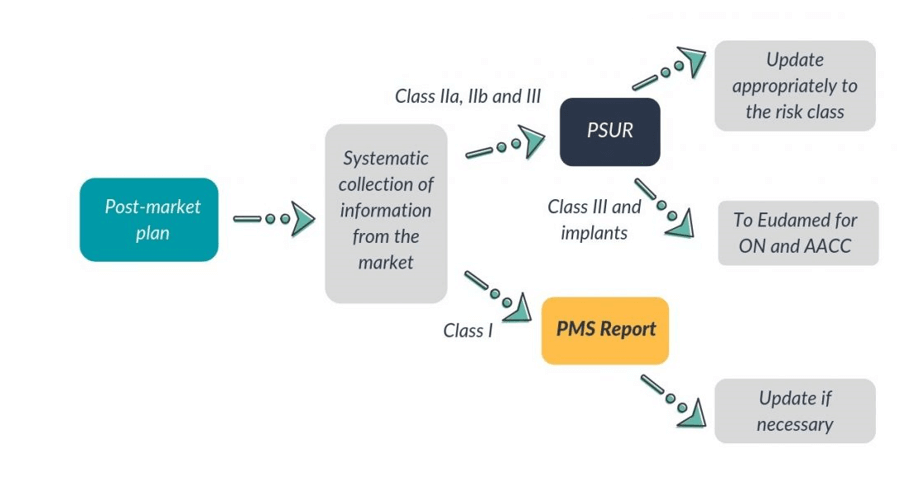
Post-market surveillance (PMS) is a system that gives continuous feedback about the performance of devices on the market in order to maintain a high standard of product quality. It is an essential part of regulatory requirements and the Medical Device Regulation (EU MDR) and In-Vitro Diagnostic Regulation (IVDR) give special importance to gathering clinical and safety-related data after the approval/CE certification process and market access. The data gathered from effective Post-market surveillance acts as critical input into the update of many technical documents like Clinical evaluation, Risk analysis, usability, etc.
Manufacturers of MD and IVD devices must have an appropriate system for gaining and reviewing experience in the post-production phase from the range of devices he manufactures. NB has to audit to verify the effectiveness of the Post-market surveillance (PMS) system. Such Post-market surveillance systems are an essential part of a manufacturer’s quality assurance system. In most cases, Post-market surveillance (PMS) systems already exist to meet internal company needs, as an integrated part of a manufacturer’s quality system, and/or to meet the requirements of third parties. In the absence of an approved quality system, the manufacturer is still required to have effective Post-market surveillance (PMS) system in place