NIOSH Certification Guide
Before moving ahead with your idea, answering these questions will help you as a respirator manufacturer to pursue and continue the road that leads to success.
- What options are available to help me increase the reliability, and maintain the regulatory requirements in the manufacturing of N95 respirators, and launching the product in the US market?
- Does the company contain any intellectual and human resources needed to bring the product to the US market?
Your solution to the above questions can be outsourcing the regulatory hassles to Maven and let the experts make your dream come true.
WHAT is NIOSH?
NIOSH stands for National Institute for Occupational Health and Safety. NIOSH is part of the U.S. Centers for Disease Control and Prevention, in the U.S. Department of Health and Human Services. It overlooks the approval and certification of Personal Protective Equipment such as N95 respirators that are used by the workers of all fields in the United States. Thus, its goal is to assure that every working man and woman in the U.S. have safe working conditions and preservation of human resources is maintained.
WHY NIOSH Approval?
Launching your N95 mask in the U.S. can only be possible through NIOSH certification. If the respirator meets the standard requirements of NIOSH, the manufacturer receives a testing and certification number, which can then be used on the product label along with the NIOSH logo.
Any respirator or respiratory protective product must be NIOSH certified before it can be used in any workplace: medical, industrial, educational, etc. The regulation that empowers NIOSH to regulate and certify respiratory protective products is 42 CFR Part 84.
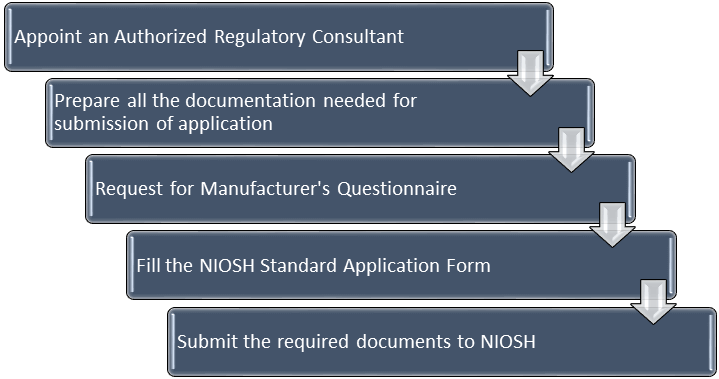
How to apply for NIOSH Certification?
Before applying for NIOSH Certification, make sure you comply to the requirements set forth in:
- Title 42 CFR Part 84
- The Standard Application Procedure for the Approval of Air-Purifying Filtering Facepiece Respirators Under 42 CFR Part 84
Did we lose you? Click here to watch a video.
How will Maven be a part of your journey?
Maven will help you in fulfilling all the steps as discussed above. Our experts suggest that implementing a Quality Management System beforehand can make the NIOSH certification process up to 2 times faster.
Maven helps you to prepare the 2 main components of the Application:
- Pre-submission Test Data: This includes the testing of your product at a Certified Testing Laboratory. This step is of utmost importance. Our experts will guide you for:
- What tests should be conducted?
- Identifying which labs have the certifications to conduct the required tests?
- Filling the sample submission form for the respective testing laboratory
- Shipping of samples to the laboratory
- Documentation: Referring to the NIOSH Standard Application Process, NIOSH requires the applicant to submit a set of required documents as follows:
- Quality Assurance Manual
- Product Quality Plan and Manufacturing Flowchart
- Matrix, Draft Label ,and User Instructions
Experts at Maven can help you prepare accurate documents according to the exact guidelines for NIOSH Approval.
Marketing an N95 respirator may be financially rewarding while also addressing complex day-to-day health challenges in these unprecedented times. The procedure should not be rushed, since the authorities will be looking for adherence to regulations and other criteria.
If you’ve followed the steps outlined here, you’ll be well on your way to bringing an N95 respirator to market in a safe and effective manner. Maven Profcon Services LLP is able to externalize your regulatory requirements, allowing you to focus on marketing and advertising.
Frequently Asked Questions
- Are there any relaxations/exceptions for NIOSH approval owing to the COVID-19 pandemic?
There are guidance documents available at https://www.cdc.gov/niosh/npptl/ regarding the NIOSH Approval process of the current pandemic situation. NIOSH is prioritizing the applicants from within the U.S. and certain other countries. To figure out if you are on the list of prioritized countries, click here or send us an email with your product details here.
- Can my respirator be used in healthcare settings?
Under the new Surgical N95 guideline from NIOSH, a surgical N95 respirator is a NIOSH-certified N95 respirator which is simultaneously approved under FDA- 510(K) as a Class II medical device. This respirator can then be sold under medical claims, for use in clinical set-ups.
- Can I get my respirator approved, if my company is not based in the US?
Yes, it is possible
- Will there be an on-site audit?
Yes
- If my respirator has achieved NIOSH certification, how will it be identified?
After NIOSH approval, the N95 respirator is supposed to be marked with the NIOSH logo and the Certification number. Simultaneously, the label is supposed to contain the NIOSH and DHHS Logos. Please note that it is against the law to use the logo before you receive your NIOSH approval.
- In which language should the documents be submitted?
All the documents must be submitted in English.
- How many samples shall be required for pre-testing?
26 samples of each type of respirator will be tested.
- What tests need to be performed?
Usually, tests for filtration efficiency and breathing resistance are performed. Other tests can be determined depending on the type of your respirator. To know more about which tests are to be conducted, send us an email with your product details here.