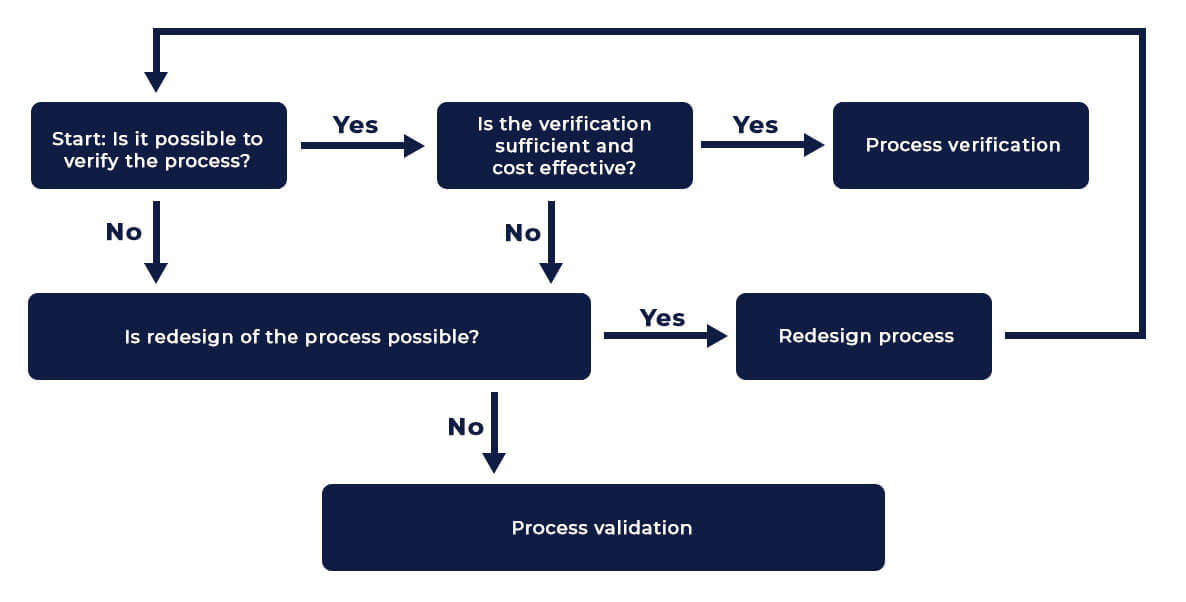
There are number of processes aligned one after another in the manufacturing flow chart of a medical device. However not all the processes are validated! Why? Let us get to the answer by understanding the terms Validation and Verification of Process.
What is Verification and Validation in Medical Device Manufacturing?
Verification and Validation of processes both ensure that the process output aligns with defined specifications. The distinction lies in how the output is assessed. When the process output can be directly monitored or measured, it is termed **process verification**. This involves checks to confirm that results meet specifications immediately.
However, when the process output cannot be directly verified through subsequent monitoring or measurement—and issues like deviations or inconsistencies become apparent only after the product is in use—it necessitates **process validation**. Validation demonstrates, through documented evidence, that the process can reliably produce the desired output consistently over time, ensuring quality and compliance.
Differences Between Verification and Validation
| Aspect | Verification | Validation |
| Focus | Accuracy of development or manufacturing processes. | Suitability and effectiveness in real-world use. |
| Stage | Performed during or after development stages. | Performed at the end of development or during use. |
| Methodology | Reviews, inspections, and testing against design inputs. | Testing in real-world or simulated environments. |
| Outcome | Ensures compliance with technical specifications. | Confirms that user needs and regulatory requirements are met. |
Regulatory Relevance | Ensures compliance with regulatory and internal standards. | Ensures safe and effective performance for end-users. |
Example of Verification: Cutting Metal Sheets
Let’s clear the concept with an example of manufacturing process of Medical device (Metal implant):
Cutting of Metal sheet is a process used to cut the sheet to desired shape using Laser source. Here the desired output is dimensions of the cut sheet. These dimensions can be measured using the Vernier caliper or Calibrated Scale or if the dimension is too small they can be measured using Microscope with software for dimension measurement. Here this measurement of dimension against the desired specification is called Verification. That is, you are verifying the output against the desired specifications.
Suggested Read:
Software Validation
Example of Validation
A medical device manufacturer is developing an automated blood glucose monitoring system. Before launching the product, the company must validate its performance to ensure it meets regulatory and quality standards.
Steps in Validation:
1. Design Validation:
– The company conducts clinical trials to verify that the glucose monitor provides accurate readings compared to a gold-standard laboratory test.
– User feedback is collected to ensure the device is easy to operate and meets patient needs.
2. Process Validation:
– Manufacturing processes are tested to confirm that every batch of devices maintains consistent quality.
– Environmental conditions (e.g., temperature, humidity) are controlled to ensure reliable sensor performance.
3. Software Validation:
– The embedded software is tested to confirm accurate calculations and data storage.
– Simulated use-case scenarios are run to check for errors or malfunctions.
4. Sterilization Validation:
– If the device includes disposable test strips, sterilization methods (e.g., gamma radiation) are validated to ensure they eliminate contaminants without damaging the product.
5. Final Validation Report:
– A validation report is compiled, summarizing all tests, results, and corrective actions.
– Regulatory bodies (such as the FDA) review the report to grant market approval.
This validation ensures the device is safe, effective, and reliable, preventing product failures and regulatory non-compliance.
Similarly Annealing (Heat treatment process) is a process in which the properties of metal like tensile strength & flexibility are achieved by raising the temperature of the metal to extreme limit and then bringing the temperature down to a specific degree. Now how do we measure that the tensile property of the metal is achieved as per the defined specification? Here we need to perform destructive tensile testing i.e. in order to measure the tensile strength, the metal will be gripped from two ends and stretched until it breaks in two parts. So, if the manufacturer will check the tensile strength by breaking each and every medical device produced, what is he/she going to sell in the market?
The Role of Verification Versus Validation Testing
Process Validation is a method in which Statistical Techniques/Procedures are used to prove that the process produces output as per the specifications consistently. Hence during the manufacturing process there is no need of performing destructive testing on each of the device manufactured.
Role of Verification Testing
Verification testing ensures that the product or process meets the specified design requirements and standards. It is conducted during the development phase and focuses on building the product right.
- Purpose: To confirm that design outputs align with design inputs.
- Methods:
– Unit and integration testing.
– Inspections, walkthroughs, and technical reviews.
– Functional, performance, and safety testing.
- Key Role: Identifies issues early in development, reducing risks and costs.
- Example: Verifying that the dimensions of a medical device meet specified tolerances.
Role of Validation Testing
Validation testing ensures that the final product fulfills its intended purpose and user needs in real-world conditions. It focuses on building the right product.
Verification testing ensures technical correctness, while validation testing ensures user relevance and product efficacy. Together, they mitigate risks, ensure compliance, and safeguard the product’s quality and usability.
What are the FDA requirements for verification and validation?
The U.S. Food and Drug Administration (FDA) requires Verification and Validation (V&V) to ensure medical devices meet design, safety, and performance standards before market approval. These requirements are outlined in 21 CFR Part 820 (Quality System Regulation – QSR).
1. Verification: Confirms that the device meets specified design requirements.
– Conducted through inspections, testing, and analysis.
– Examples: Software testing, component inspections, and dimensional analysis.
2. Validation: Ensures the device meets user needs and intended use.
– Requires clinical evaluations, simulated use testing, and risk assessments.
– Examples: Clinical trials, usability testing, and process validation.
Key FDA Expectations:
– Design Controls (21 CFR 820.30) – Requires documented verification and validation.
– Risk Management – Devices must comply with ISO 14971 for risk assessment.
– Process Validation (21 CFR 820.75) – Manufacturing processes must consistently produce quality products.
FDA compliance ensures medical devices are safe, effective, and regulatory-approved before reaching the market.
Challenges in Verification and Validation
Challenges in Verification
1.Incomplete or Ambiguous Requirements:
Difficulty arises when requirements are not clearly defined, making it challenging to verify if design outputs meet expectations.
2.Complexity of Systems:
Modern medical devices often involve complex hardware, software, and integration, making verification resource-intensive and error-prone.
3.Limited Resources:
Tight project budgets or limited personnel can lead to incomplete testing or missed verification steps.
4.Lack of Traceability:
Difficulty in maintaining a clear link between requirements, design outputs, and verification activities.
5.Changing Requirements:
Frequent changes to specifications can disrupt verification activities, requiring rework and additional resources.
Challenges in Validation
1.Simulating Real-World Conditions:
Recreating realistic operational environments can be costly and complex, especially for devices with diverse use cases.
2.Limited Access to End-Users:
Engaging clinicians, patients, or other users for validation testing can be difficult, leading to limited feedback.
3.Regulatory Compliance:
Adhering to stringent validation standards (e.g., FDA, ISO 13485) while balancing time and cost constraints is a common challenge.
4.Long Validation Cycles:
Some processes, such as clinical trials, take extended periods, delaying product launch.
5.Measurement Limitations:
For processes where outputs cannot be directly measured or monitored, proving consistent performance can be challenging.
Best Practices for Effective Verification and Validation
1.Clear Requirements: Define measurable, unambiguous requirements early.
2.Verification and Validation Plan: Develop a detailed plan with objectives, methods, and success criteria.
3.Risk-Based Approach: Prioritize testing for high-risk components.
4.Early Integration: Start V&V in the design phase for early issue identification.
5.Automation: Use automated tools for efficiency in testing and documentation.
6.Cross-Functional Collaboration: Involve engineering, quality, and end-users in V&V activities.
7.Real-World Simulation: Test under conditions mimicking actual usage.
8.Comprehensive Documentation: Maintain detailed records of V&V activities.
9.Continuous Monitoring: Gather feedback and monitor post-validation performance.
By following these best practices, organizations can streamline their Verification and Validation processes, mitigate risks, and ensure that medical devices meet both technical specifications and user needs.
How can Maven help?
For processes where outputs cannot be directly measured or monitored, proving consistent performance can be challenging.
Maven helps streamline regulatory compliance, quality management, and audits by automating documentation, ensuring FDA and ISO 13485 compliance, and optimizing verification and validation processes for medical device manufacturers.
Conclusion
Maven simplifies regulatory compliance by automating quality management, streamlining verification and validation, and ensuring adherence to FDA, ISO 13485, and MDSAP standards. By reducing audit burdens and improving efficiency, Maven helps medical device manufacturers enhance product quality, accelerate market approvals, and maintain compliance, ultimately ensuring patient safety and regulatory success.