Unique Device Identifier for medical devices defined as a series of numeric or alphanumeric characters that is created through internationally accepted device identification and coding standards and that allows unambiguous identification of specific devices on the market.
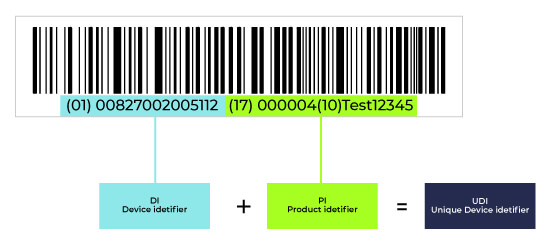
The UDI for medical devices shall be used for reporting serious incidents and field safety corrective actions in accordance with Article 87.The UDI-DI is the device identifier. It identifies a specific device on your portfolio. This is the static part of the UDI number. It doesn´t change within the same exact product. But we´ll go more deeply on that on the next chapters.
The UDI-PI is the production identifier. It is the dynamic part of the UDI. It tells you about lot number, serial number, manufacturing date, expiration date.
But before that, you need the Basic UDI-DI which identifies a group of product. It´s different from the UDI-DI.
Regulatory Requirements for UDI Documentation in Annex II
As part of the technical documentation referred to in Annex II, the manufacturer shall keep up-to-date a list of all UDIs that it has assigned. 5.5.2017 L 117/35 Official Journal of the European Union EN.
Economic operators shall store and keep, preferably by electronic means, the UDI for medical devices which they have supplied or with which they have been supplied, if those devices belong to:
- class III implantable devices;
- The devices, categories or groups of devices determined by a measure referred to in point (a) of paragraph 11.
As per Article 29 Registration of devices, before placing a device, other than a custom-made device, on the market, the manufacturer shall, in accordance with the rules of the issuing entity referred to in Article 27(2), assign a Basic UDI-DI as defined in Part C of Annex VI to the device and shall provide it to the UDI database together with the other core data element By the dates that you will see in the chapter “UDI transition period” you´ll need to place the UDI carrier on the label of the device and on all higher levels packaging.
But there are certain cases where it can be different.
For example, for class I and IIa single-use devices, packaged and labeled individually. In that case, the UDI code can be placed not directly on the primary packaging, but on the next higher packaging. This packaging can contain several of these individual package products.
For reusable devices, the UDI should be directly marked on the product. It´s true that there are few exceptions (If space doesn´t allow that for example).
For this last case, the EU is different than the US. FDA authorize to have the AIDC or the HRI. But in the EU both should be visible. And it is referred to in Part B of Annex VI related to that device.
The natural or legal person responsible shall assign to the system or procedure pack, in compliance with the rules of the issuing entity, a Basic UDI-DI and shall provide it to the UDI database together with the other core data elements referred to in Part B of Annex VI related to that system or procedure pack.
As per Part C of Annex VI:
General requirements
The affixing of the UDI for medical devices is an additional requirement — it does not replace any othermarking or labelling requirements laid down in Annex I to this Regulation.
The manufacturer shall assign and maintain unique UDI for medical devices.
The UDI for medical devices may placed on the device or its packaging by the manufacturer.
Only coding standards provided by issuing entities designated by the Commission pursuant to Article 27(2) may be used.
Explore Our Services:
Medical Device Labelling
The UDI for Medical device
A UDI for medical devices shall be assigned to the device itself or its packaging. Higher levels of packaging shall have their own UDI.
Shipping containers shall be exempted from the requirement in Section 3.1. By way of example, UDI for medical devices shall not be required on a logistics unit; where a healthcare provider orders multiple devices using the UDI or model number of individual devices and the manufacturer places those devices in a container for shipping or to protect the individually packaged devices, the container (logistics unit) shall not be subject to UDI requirements.
Components of Unique Device Identifier
The UDI for medical devices shall contain two parts: a UDI-DI and a UDI-PI.
The UDI-DI shall be unique at each level of device packaging.
If a lot number, serial number, software identification or expiry date appears on the label, it shall be part of the UDI-PI. If there is also a manufacturing date on the label, it does not need to be included in the UDI-PI. If there is only a manufacturing date on the label, this shall be used as the UDI-PI.
Each component that is considered to be a device and is commercially available on its own shall be assigned a separate UDI unless the components are part of a configurable device that is marked with its own UDI.
Systems and procedure packs as referred to in Article 22 shall be assigned and bear their own UDI.
The manufacturer shall assign the UDI to a device following the relevant coding standard.
A new UDI-DI shall be required whenever there is a change that could lead to misidentification of the device and/or ambiguity in its traceability; in particular, any change of one of the following UDI database data elements shall require a new UDI-DI:
- name or trade name,
- device version or model,
- labelled as single use,
- packaged sterile,
- need for sterilization before use,
- quantity of devices provided in a package,
- critical warnings or contra-indications: e.g. containing latex or DEHP.
Manufacturers that repackage and/or relabel devices, with their own label shall retain a record of the original device manufacturer’s UDI.
UDI carrier
The UDI carrier (AIDC and HRI representation of the UDI) shall be placed on the label or on the device itself and on all higher levels of device packaging. Higher levels do not include shipping containers.
In the event of there being significant space constraints on the unit of use packaging, the UDI carrier may be placed on the next higher packaging level.
For single-use devices of classes I and IIa packaged and labelled individually, the UDI carrier shall not be required to appear on the packaging but it shall appear on a higher level of packaging, e.g. a carton containing several individually packaged devices. However, when the healthcare provider is not expected to have access, in cases such as in home healthcare settings, to the higher level of device packaging, the UDI shall be placed on the packaging of the individual device.
For devices exclusively intended for retail point of sale the UDI-PIs in AIDC shall not be required to appear on the point of sale packaging.
When AIDC carriers other than the UDI carrier are part of the product labelling, the UDI carrier shall be readily identifiable.
If linear bar codes are used, the UDI-DI and UDI-PI may be concatenated or non-concatenated in two or more bar codes. All parts and elements of the linear bar code shall be distinguishable and identifiable.
If there are significant constraints limiting the use of both AIDC and HRI on the label, only the AIDC format shall be required to appear on the label. For devices intended to be used outside healthcare facilities, such as devices for home care, the HRI shall however appear on the label even if this results in there being no space for the AIDC.
The HRI format shall follow the rules of the UDI code-issuing entity.
If the manufacturer is using RFID technology, a linear or 2D bar code in line with the standard provided by the issuing entities shall also be provided on the label.
Devices that are reusable shall bear a UDI carrier on the device itself. The UDI carrier for reusable devices that require cleaning, disinfection, sterilisation or refurbishing between patient uses shall be permanent and readable after each process performed to make the device ready for the subsequent use throughout the intended lifetime of the device. The requirement of this Section shall not apply to devices in the following circumstances:
- any type of direct marking would interfere with the safety or performance of the device;
- the device cannot be directly marked because it is not technologically feasible.
The UDI carrier shall be readable during normal use and throughout the intended lifetime of the device.
If the UDI carrier is readily readable or, in the case of AIDC, scanable, through the device’s packaging, the placing of the UDI carrier on the packaging shall not be required.
In the case of single finished devices made up of multiple parts that must be assembled before their first use, it shall be sufficient to place the UDI carrier on only one part of each device.
The UDI carrier shall be placed in a manner such that the AIDC can be accessed during normal operation or storage.
Bar code carriers that include both a UDI-DI and a UDI-PI may also include essential data for the device to operate or other data.
General principles of the UDI database
The UDI database shall support the use of all core UDI database data elements referred to in Part B of this Annex.
Manufacturers shall be responsible for the initial submission and updates of the identifying information and other device data elements in the UDI database.
Appropriate methods/procedures for validation of the data provided shall be implemented.
Manufacturers shall periodically verify the correctness of all of the data relevant to devices they have placed on the market, except for devices that are no longer available on the market.
The presence of the device UDI-DI in the UDI database shall not be assumed to mean that the device is in conformity with this Regulation.
The database shall allow for the linking of all the packaging levels of the device.
The data for new UDI-DIs shall be available at the time the device is placed on the market.
Manufacturers shall update the relevant UDI database record within 30 days of a change being made to an element, which does not require a new UDI-DI.
Internationally-accepted standards for data submission and updates shall, wherever possible, be used by the UDI database.
The user interface of the UDI database shall be available in all official languages of the Union. The use of free- text fields shall, however, be minimized in order to reduce translations.
Data relating to devices that are no longer available on the market shall be retained in the UDI database.
Cases and Exemptions in UDI Marking Requirements
Implantable devices:
Implantable devices shall, at their lowest level of packaging (‘unit packs’), be identified, or marked using AIDC, with a UDI (UDI-DI + UDI-PI);
The UDI-PI shall have at least the following characteristics:
(a) the serial number for active implantable devices,
(b) the serial number or lot number for other implantable devices.
The UDI of the implantable device shall be identifiable prior to implantation.
Reusable devices requiring cleaning, disinfection, sterilisation or refurbishing between uses.
The UDI of such devices shall be placed on the device and be readable after each procedure to make the device ready for the next use.
The UDI-PI characteristics such as the lot or serial number shall be defined by the manufacturer.
Systems and procedure packs as referred to in Article 22.
The natural or legal person referred to in Article 22 shall be responsible for identifying the system or.
procedure pack with a UDI including both UDI-DI and UDI-PI.
Device contents of system or procedure packs shall bear a UDI carrier on their packaging or on the device itself.
Exemptions:
- individual single-use disposable devices, the uses of which are generally known to the persons by whom they are intended to be used, which are contained within a system or procedure pack, and which are not intended for individual use outside the context of the system or procedure pack, shall not be required to bear their own UDI carrier;
- devices that are exempted from bearing a UDI carrier on the relevant level of packaging shall not be required to bear a UDI carrier when included within a system or procedure pack.
Placement of the UDI carrier on systems or procedure packs
- The system or procedure pack UDI carrier shall as a general rule be affixed to the outside of the packaging.
- The UDI carrier shall be readable, or, in the case of AIDC, scannable, whether placed on the outside of the packaging of the system or procedure pack or inside transparent packaging.
Configurable devices:
A UDI shall be assigned to the configurable device in its entirety and shall be called the configurable device UDI.
The configurable device UDI-DI shall be assigned to groups of configurations, not per configuration within the group. A group of configurations is defined as the collection of possible configurations for a given device as described in the technical documentation.
A configurable device UDI-PI shall be assigned to each individual configurable device.
The carrier of the configurable device UDI shall be placed on the assembly that is most unlikely to be exchanged during the lifetime of the system and shall be identified as the configurable device UDI.
Each component that is considered a device and is commercially available on its own shall be assigned a separate UDI.
Device Software
UDI assignment Criteria.
The UDI shall be assigned at the system level of the software. Only software which is commercially available on its own and software which constitutes a device in itself shall be subject to that requirement.
The software identification shall be considered to be the manufacturing control mechanism and shall be displayed in the UDI-PI.
A new UDI-DI shall be required whenever there is a modification that changes:
- the original performance;
- the safety or the intended use of the software;
- interpretation of data.
Such modifications include new or modified algorithms, database structures, operating platform, architecture or new user interfaces or new channels for interoperability.
Minor software revisions shall require a new UDI-PI and not a new UDI-DI.
Minor software revisions are generally associated with bug fixes, usability enhancements that are not for safety purposes, security patches or operating efficiency.
Minor software revisions shall be identified by a manufacturer-specific form of identification.
UDI placement criteria for software
where the software is delivered on a physical medium, e.g. CD or DVD, each packaging level shall bear the human readable and AIDC representation of the complete UDI. The UDI that is applied to the physical medium containing the software and its packaging shall be identical to the UDI assigned to the system level software;
the UDI shall be provided on a readily accessible screen for the user in an easily-readable plain-text format, such as an ‘about’ file, or included on the start-up screen;
software lacking a user interface such as middleware for image conversion, shall be capable of transmitting the UDI through an application programming interface (API);
only the human readable portion of the UDI shall be required in electronic displays of the software. The marking of UDI using AIDC shall not be required in the electronic displays, such as ‘about’ menu, splash screen etc.;
the human readable format of the UDI for the software shall include the Application Identifiers (AI) for the standard used by the issuing entities, so as to assist the user in identifying the UDI and determining which standard is being used to create the UDI.
Ensuring UDI Compliance with Maven Profcon’s Advanced Solutions
Maven Profcon Services LLP simplifies UDI compliance for medical devices, including software. Our solutions focus on integrating UDIs seamlessly into software interfaces like ‘about’ screens and ensuring API compatibility for middleware. With our expertise, meeting EU MDR and global UDI requirements is straightforward and efficient.