Required Documents for Medical Devices Registration in Sri Lanka
- Request letter for registration: A formal request letter addressed to the regulatory authority.
- Business registration certificate: Proof of the manufacturer’s legal entity in their home country.
- Authorization Letter: Authorizing a local representative or agent to act on behalf of the manufacturer.
- Free Sales Certificate: Confirming that the medical device is freely sold in the manufacturer’s home country.
- Sample license: License permitting the importation of sample devices for registration purposes.
- Certificate from the manufacturing country: Attesting to the device’s compliance with regulatory standards in the manufacturing country.
- List of countries with prior approval: Details of countries where the device has already been approved for sale.
- Registration Dossier: Comprehensive documentation outlining the device’s specifications, safety, and performance.
- Product samples: Actual samples of the medical device for evaluation purposes.
- Labels, IFUs: Labeling and Instructions for Use (IFUs) in compliance with Sri Lankan regulations.
- Test Reports, Clinical Studies Reports: Reports demonstrating the device’s safety and efficacy through testing and clinical studies.
- QMS certificate: Certificate confirming adherence to Quality Management System (QMS) standards.
- CE certificate: Certificate indicating compliance with European Union (EU) regulations, if applicable.
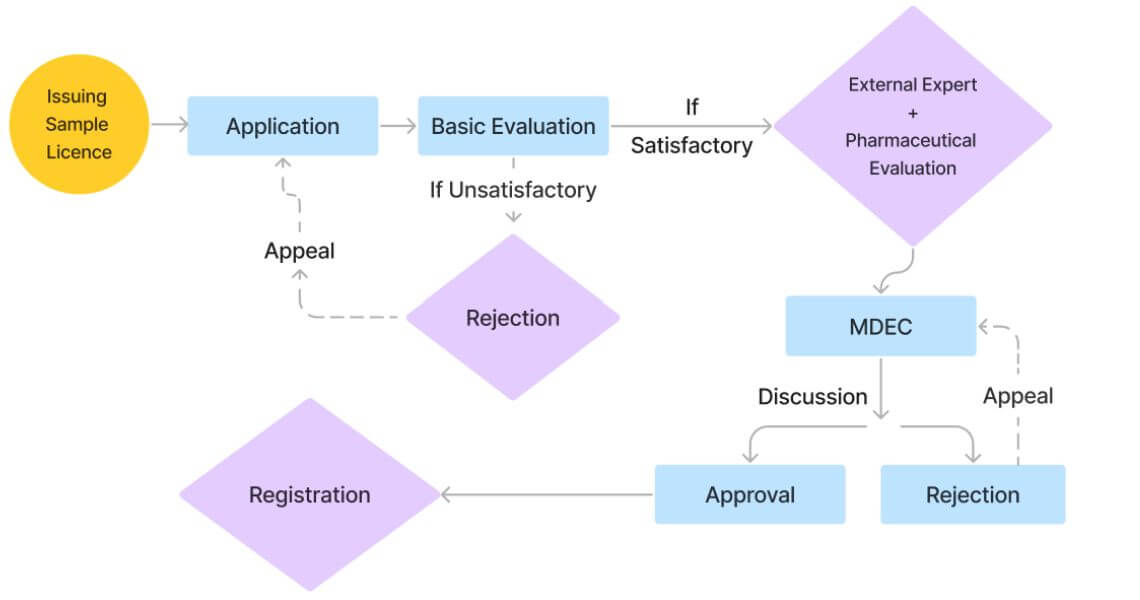
Ensuring all necessary documents are prepared and submitted accurately is essential for a smooth and successful registration process for medical devices in Sri Lanka.
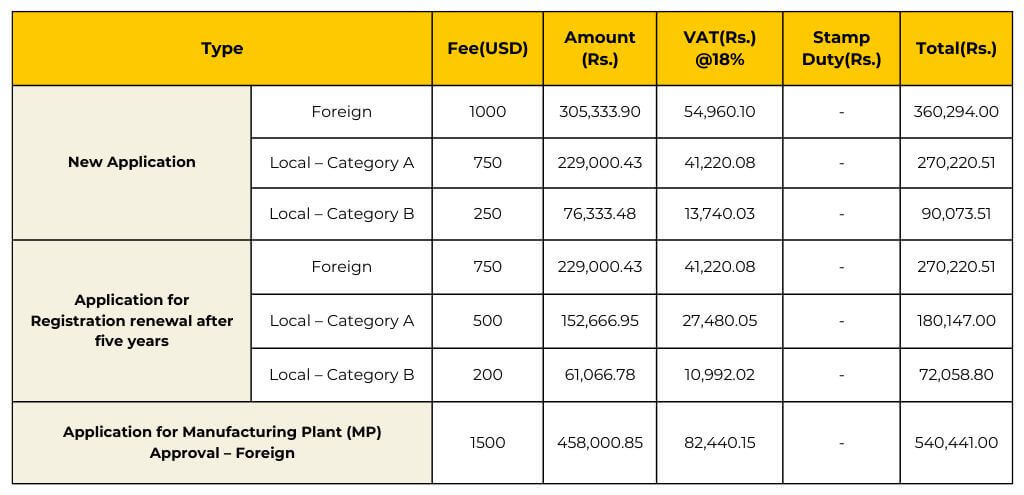
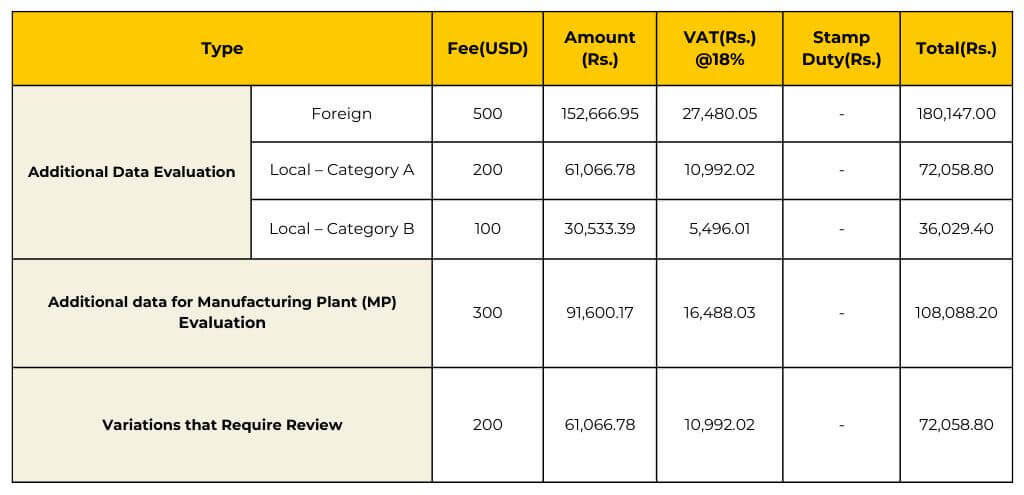
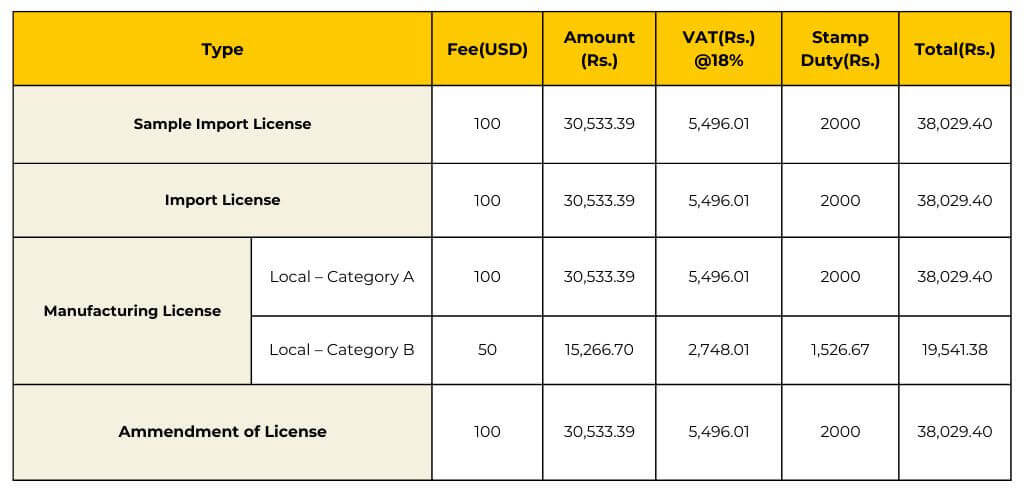
Ensure compliance with Sri Lanka’s regulations by obtaining the appropriate medical devices registration licenses. The validity periods vary depending on the type of license, ranging from 1 to 5 years.
Special Requirements
- Chemical, Physical, and Biological Properties: Medical devices must meet stringent standards regarding their chemical, physical, and biological properties to safeguard patient health.
- Infection Risk Minimization: Measures should be in place to minimize the risk of infection and microbial contamination to patients, users, and other individuals.
- Safety in Construction and Environment: Devices must demonstrate safe construction and environmental properties to mitigate potential hazards.
- Measurement Accuracy: Medical devices with measuring functions should deliver precise and stable measurements to ensure reliable performance.
- Radiation Protection: Adequate protection against radiation is essential to safeguarding the health and safety of patients, users, and others.
- Energy Source Reliability: Devices connected to or equipped with energy sources must be designed and produced to ensure optimal performance, reliability, and repeatability while minimizing risks associated with potential faults.
Compliance with these special requirements is critical for manufacturers seeking medical device registration in Sri Lanka. By prioritizing safety and quality in device design and production, companies can ensure regulatory compliance and enhance confidence in their products within the Sri Lankan market.
Entrust your medical devices registration process in Sri Lanka to Maven, where expertise meets efficiency. With our comprehensive services, navigating the intricacies of medical devices registration becomes seamless. Benefit from our tailored solutions designed to ensure compliance with regulatory standards in Sri Lanka, allowing you to focus on your core business objectives. Partner with Maven for a smooth and successful registration process, ensuring your medical devices gain market access and meet all requirements in Sri Lanka.