Healthcare in the Kingdom of Saudi Arabia (KSA) holds significant importance, with a rising demand for medical devices across the nation. The Saudi Food and Drug Authority (SFDA), under the purview of the Council of Ministers, oversees the registration of medical devices in Saudi Arabia. Compliance with Saudi Arabia’s Medical Device Regulations is essential, necessitating the appointment of a Saudi Arabia-authorized representative.
However, navigating the regulatory landscape in Saudi Arabia can present challenges. Cultural inclinations, linguistic barriers, and the rigorous scrutiny imposed by regulatory authorities can pose significant hurdles in the medical device registration process. Additionally, obtaining cooperation from the agency may sometimes prove challenging, further complicating the path to device approval.
Despite these challenges, ensuring compliance with Saudi Arabia’s regulatory requirements for medical device registration is crucial for accessing the burgeoning healthcare market in the Kingdom. Companies looking to expand their presence in Saudi Arabia must carefully navigate the regulatory landscape and address the complexities inherent in the registration process.
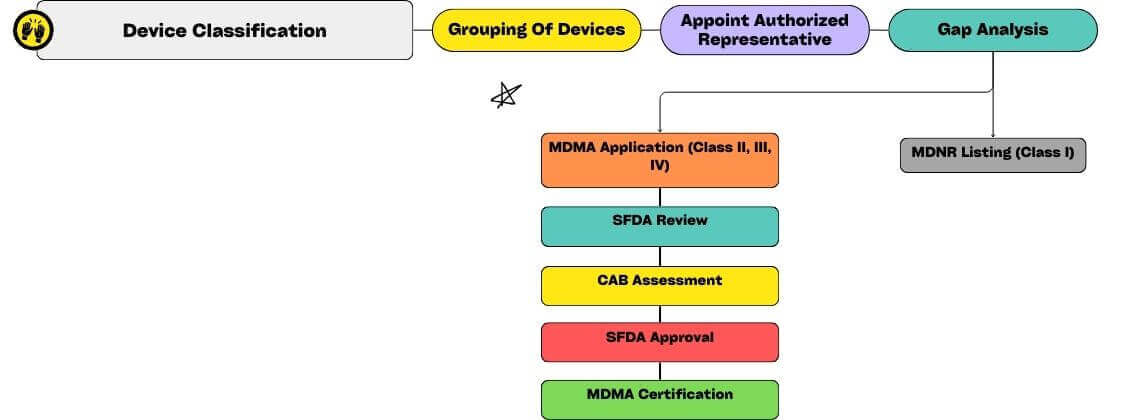
Saudi Arabian Medical Device Classification
- Classification Criteria: Medical devices are categorized into four classes in Saudi Arabia. This classification includes grouping models, variants, and accessories within a single application.
- Formal Classification: For borderline products or those falling under different classes in reference countries, the Saudi Food and Drug Authority (SFDA) allows formal classification.
- Compliance Requirements: Companies exporting medical devices to Saudi Arabia must ensure compliance with the classification criteria outlined by SFDA.
- Registration Process: Once classified, medical devices must undergo the registration process with the SFDA to obtain market authorization for distribution in Saudi Arabia.
- Renewal and Surveillance: Registered devices require periodic renewal to maintain compliance with regulatory standards. Additionally, post-market surveillance is essential to monitor device safety and performance.
Saudi Arabia’s Authorized Representative
| Device Class as per KSA | Approval routes | Timelines | Validity | Device Class as per EU MDR |
| Class A | MDNR Listing | 1 Month | 3 years | Class I |
| Class A – Sterile | MDNR Listing | 1 Month | 3 years | Class I – s |
| Class A – Measuring Function | MDNR Listing | 1 Month | 3 years | Class I – m |
| Class A – Reusable Surgical Instruments | MDNR Listing | 1 Month | 3 years | Class I – r |
| Class B | MDMA Approval | 6 Months | Depends on validity of Original License | Class IIa |
| Class C | MDMA Approval | 9-24 Months | Depends on validity of Original License | Class IIb |
| Class D | MDMA Approval | 9-24 Months | Depends on validity of Original License | Class III |
In Saudi Arabia, foreign manufacturers seeking to market medical devices must appoint a Medical Device Authorized Representative (AR) to facilitate the registration process. This AR entity, licensed through the Medical Device Establishment (MDEL) System, serves as the intermediary between the manufacturer and Saudi regulatory authorities.
The role of the Authorized Representative is pivotal in ensuring compliance with Saudi Arabia’s regulations governing medical devices. They act as a liaisons, facilitating communication and documentation exchange between the manufacturer and regulatory authorities. Additionally, the AR entity assumes responsibility for ensuring that the medical devices meet all regulatory requirements before they can be marketed in Saudi Arabia.
Having a reputable and licensed Authorized Representative is essential for foreign manufacturers looking to navigate the complex landscape of medical device registration in Saudi Arabia. By entrusting this responsibility to a qualified entity, manufacturers can streamline the registration process and ensure timely market access for their products.
Registration Costs
Local Fees (New Application)
| Service | Fees (USD) |
| Class A/General IVDs/Exempt IVD (TGA) | $4000 |
| Class B/Self-test IVD/Listable IVD | $5067 |
| Class C/Class III (CA/Annex II List B) (IVD) | $5600 |
| Class D/AIMD/Annex II List A (IVD)/Registrable IVD | $6133 |
| Medical Device Listing in Medical Device National Registry (MDNR) | $135 |
*Please note that these are fees currently enforced. However, as soon as the new regulation is enforced, the new fee schedule will be implemented.
Local Fees (Manufacturer)
| Service | Fees (USD) |
| Importation and/ or distribution of medical devices (varies) | From $4000 to $9350 |
| License for Authorized Representative (AR) | $1300 per year |
Saudi Arabia Medical Device Registration
Saudi Arabia imposes specific requirements for the registration of medical devices, which vary depending on the device’s classification. Two pathways exist for approval based on the device class:
- Medical Device National Registry (MDNR) Listing: Class I Non-sterile/ Non-Measuring Low-Risk Medical Devices necessitate listing in the MDNR before marketing in Saudi Arabia. Any entity importing or distributing such devices in the country can undertake this process. Requirements include basic product and manufacturer information, proof of Quality Management System (QMS), reference country approval, Instructions for Use (IFU), Unique Device Identification (UDI), labelling, and marketing materials. SFDA typically approves medical device registration through this pathway within 4 working days, valid for 3 years.
- Medical Device Marketing Authorization (MDMA): All other device classes must secure MDMA for market authorization in Saudi Arabia. SFDA’s medical device registration timeline for MDMA approval usually spans 35 days, with licenses valid for either the original license period or 3 years for the undefined original license validity.
These stringent registration processes ensure compliance with Saudi Arabia’s regulatory standards, enabling safe and effective medical devices to enter the market. Manufacturers and distributors should adhere to these requirements to ensure smooth market access and compliance with Saudi Arabian regulations.
Documentation requirements
Prior to being placed on the market and/ or put into service within the KSA, the device must obtain SFDA approval.
- The medical device shall comply with the Essential Principles of Safety and Performance specified in Annexes (1) and (2).
- Prepare and retain related Medical Device Technical Documentation and IVD Technical Documentation that confirm to the Essential Principles of Safety and Performance specified in Annex (3) and Annex (4), depending on the type of medical device.
- Create, document, and maintain an effective quality management system (QMS) in accordance with the international ISO standard (ISO 13485:2016) or any equivalent adopted standard for the same issue/version.
Process Flow