Government Authority
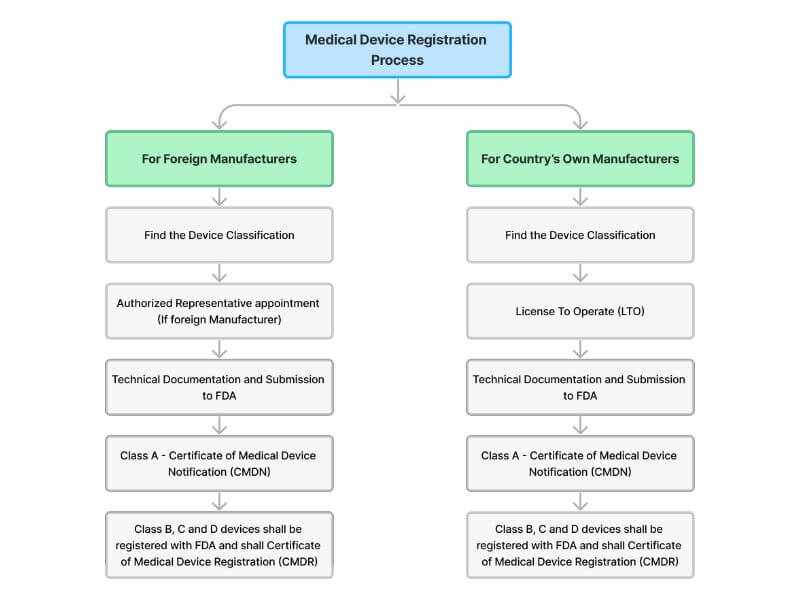
In the Philippines, oversight of medical device registration falls under the jurisdiction of the Center for Device Regulation, Radiation Health, and Research (CDRRHR) within the Department of Health. (AMDD) ASEAN Medical Device Directive provided the list of documents needed for the registration of Medical Devices as per the Classification.
The documents required for LTO for a medical device importer/distributor are:
- Business Registration Certificate
- Location Plan
- Contract of lease of Space
- Pharmacist Information
- Product Listing
- Floor Plan
- Agreement with Medical Device Manufacturer for Distribution
Time frame to obtain the LTO is between 1 – 3 months.