Who is eligible to register a medical device in Malaysia?
Only locally registered companies in Malaysia, recognized by the Companies Commission (SSM), are permitted to apply for an establishment license, proceed with medical device registration, and obtain export permits. Once registered with the SSM, companies can access MeDCASt2.0+ to initiate their application for an Establishment License, tailored to their specific activity: Manufacturer’s License, Importer’s License, Distributor’s License, or an Authorized Representative’s License. Having an Establishment License is a prerequisite for registering a medical device in Malaysia.
Since only local companies can apply for an Establishment License, foreign manufacturers have several options to register their medical devices in Malaysia:
- Establish a local subsidiary entity dedicated to registering the medical device.
- Engage a local importer or distributor possessing a valid establishment license, Good Distribution Practice for Medical Devices (GDPMD) certification, and a Registrant Account in the Medcast system to handle the registration process.
- Utilize an independent Authorized Representative like Maven for registering the medical devices. With a valid Medcast Registrant Account, Maven acts directly on behalf of foreign manufacturers, handling device registration and license maintenance. This setup offers flexibility in appointing importers or distributors without affecting the product license status amidst changes in the distribution network.
Introducing Medcast (Medical Device Centralized Online Application System)
Medcast, also known as the Medical Device Centralised Online Application System (MeDCASt), is an online platform managed by the Malaysian Medical Device Authority (MDA). It is the primary system for handling various applications related to Establishment Licensing, Medical Device Registration, and Export Permit.
Parties involved in the medical device industry in Malaysia, including manufacturers, importers, exporters, distributors, and Authorized Representatives, are required to utilize Medcast for submitting applications, especially for medical device registration with the MDA.
Originally launched in July 2013, Medcast underwent an upgrade to Medcast 2.0 in January 2019. As of February 1, 2022, MDA has introduced further enhancements with Medcast 2.0+ to improve the user experience and service quality.
Medcast 2.0+ introduces new features such as the Change Notification Module, Device Study sub-module (under the Clinical Research module), Clinical Research Use sub-module (under the Clinical Research module), and Demonstration for Marketing Notification and Payment module. These additions reflect MDA’s commitment to continuous improvement in service delivery for stakeholders in the medical device industry.
Medical device registration timeline and fees in Malaysia
| Registration Stage | Timeline | Fees (MYR) |
| Submission of Application | 2-4 weeks | 500-2000 |
| Document Preparation | 2-3 weeks | 1000-3000 |
| Compliance Assessment | 4-6 weeks | 2000-5000 |
| Inspection (if required) | 6-8 weeks | 3000-7000 |
| Review and Approval | 8-12 weeks | 5000-10000 |
| Certificate Issuance | 2-4 weeks | 1000-2000 |
| Renewal | Every 1-3 years | 2000-5000 |
| Post-Market Surveillance | Ongoing | N/A |
*NOTE – Please note that the timelines and fees provided above are approximate and may vary depending on factors such as the complexity of the medical device and the responsiveness of the regulatory authority.
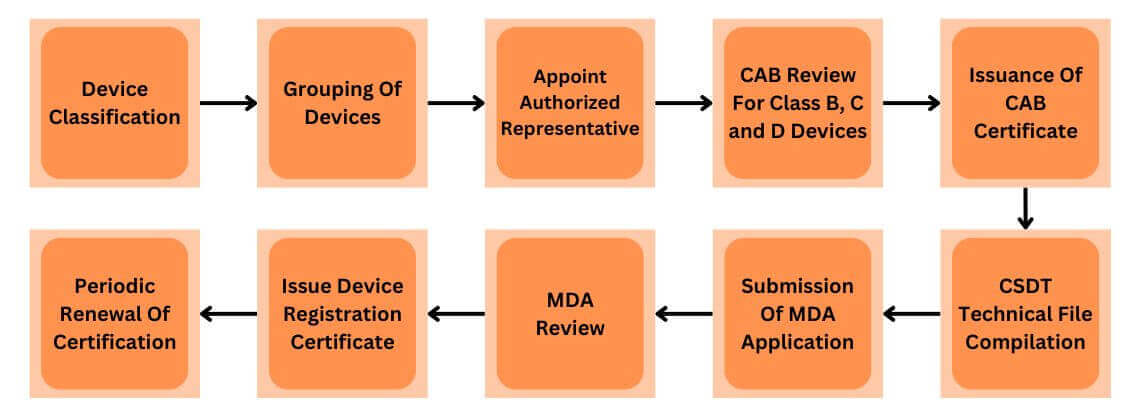
Registration Process
Registration Pathway
| Class | Registration Pathway |
| A (Low-Risk) | MDA Application |
| B (Low-Moderate) | CAB Assessment followed by MDA Application |
| C (Moderate-High) | CAB Assessment followed by MDA Application |
| D (High-Risk) | CAB Assessment followed by MDA Application |
*Note – Conformity Assessment Body
What is considered a medical device in Malaysia?
In Malaysia, the term ‘medical device’ encompasses a wide range of products utilized in healthcare for diagnosis, prevention, monitoring, or treatment purposes, excluding drugs. This definition also includes in-vitro diagnostics (IVDs). To comply with regulatory standards outlined in Section 2 of Act 737 (refer to guidance document MDA/GD/0006), any medical device must undergo registration before importation, exportation, or placement in the market.