If a problem takes place in any medical device, questions that immediately come to mind are “why, when, what, who, how, where?” and it is important to get the correct solution quickly. It will be easy to get correct answer if your product identification and traceability is well built.
Identification and traceability are the most critical terms in medical device manufacturing and very important for the medical device manufacturer to maintain the same. The Quality Management Systems built as per all global standards (Requirements stated in ISO 13485, 21 CFR Part 820, MDSAP) is built around practices that ensure Identification and Traceability at every stage of product manufacturing with an organization. Most of the non-conformities that occur in medical device manufacturing are due to lack of identification and traceability. Both processes are inter-related because only if you properly identify the system then you can easily track it.
Identification and traceability implementation in medical device life cycle
Identification is a process of showing the present status or condition of the product/material. It prevents your product from mix up and helps to easily understand the current condition of product/material. It is used at every stage of medical device and very important in supply chain. As per ISO 13485:2016 standard, “Identification of product status shall be maintained throughout production, storage, installation and servicing of product to ensure that only product that has passed the required inspections and tests, released under an authorized concession is dispatched.” Which means identification will be maintained at each stage of medical device to ensure the only product that has been confirmed by inspection and testing, will be released for dispatch. Identification can be done through various means and methods as deemed appropriate by an organization as long as it is understood by everyone in the process. The most frequently used identification is through tags and labels.
1. Identification of material at In-coming stage
When any raw material is received, identification can be given by tags or labels with raw material details like the Supplier Name, Batch No., Date of receipt, material identification number and etc. so it can be traceable. Furthermore, extra precautionary measures can be implemented by differentiating the premises and assigning an area identification i.e. Rejection area, accepted area and under test area and the raw material can be bifurcated accordingly.
Generally practiced identification tags:
Under test material (Yellow tag)
Accepted material (Green tag)
Rejected material (Red tag)
2. Identification of materials at production stage
At production stage, Identification can be given by tags which includes detail of Raw material reference Number, Date, Material Name, Name of Operation completed, Process name where further to move, Quantity moved, rework details. The containers carrying the in-process or semi-finished products are usually labelled. It is a general practice to have line-clearances between batches or products to ensure there is no mix-up. The Quality assurance person is responsible for ensuring the same.
3. Identification of product at Final packaging and labelling
Finished products should be identified by product label and placed on the device packaging or device itself at a designated area. Final labels should have all the necessary information of device like Product name and specification, lot/ batch number, sterilization details if any, Manufacturing date, expiry date and manufacturer information. The labels further carry symbols as per the international standards for labelling applicable to a particular product. If labelling is not possible directly on the device, it is recommended to have the same on the immediate packaging layer.
4. Identification of product by end user
As per Regulation of (EU) 2017/245, the traceability of medical devices shall be maintained by assigning UNIQUE DEVICE IDENTIFICATION to your product. End user can access all manufacturing information by unique device identification.
It is an Identification system for medical devices based on Unique Device Identifier. ‘Unique device identifier’ is a series of numeric or alphanumeric characters that is created through internationally accepted device identification and coding standards and that allows clear and distinct identification of medical devices on the market. It should be in Automated identification and data capture (machine readable) and clearly readable plain text (human readable). A UDI is assigned to medical device itself or all higher levels of device packaging. The UDI is comprised of the UDI-DI and UDI-PI. (UDI = UDI-DI + UDI-PI)
A. Basic UDI-DI: It is the primary identifier of a device. It is device identifier assigned at the level of device unit. It is the main key records in the UDI database. It will be given to particular medical device family which means the devices having same design and performance characteristics, same intended purpose, same risk classification and same manufacturing process. It will be referenced in relevant certificates, EU declarations of conformity and technical documentations and not appear on packaging level.
B. Device identifier (UDI-DI): It is specific to a manufacturer and a device which is used as the ‘access key’ to information stored in a UDI database.
C. Production identifier (UDI-PI): It is a numeric or alphanumeric code that identifies unit of device production. It is made of the serial number, lot number, software identification, manufacturing or expiry date or both types of date.
Let’s have a look at the Unique Device Identification system,
As indicated in this image,
-
UDI-DI (Device identifier) is the number after
(O1).
-
UDI-PI part includes Lot/batch number after
(10)
(17)
(21)
Main benefits of Unique Device Identification system:
- Excellent traceability
- Effectiveness of post market safety related activities
- Better monitoring by competent authorities
- Help to reduce medical errors and fight against falsified devices
- Customer accessible product information
Traceability
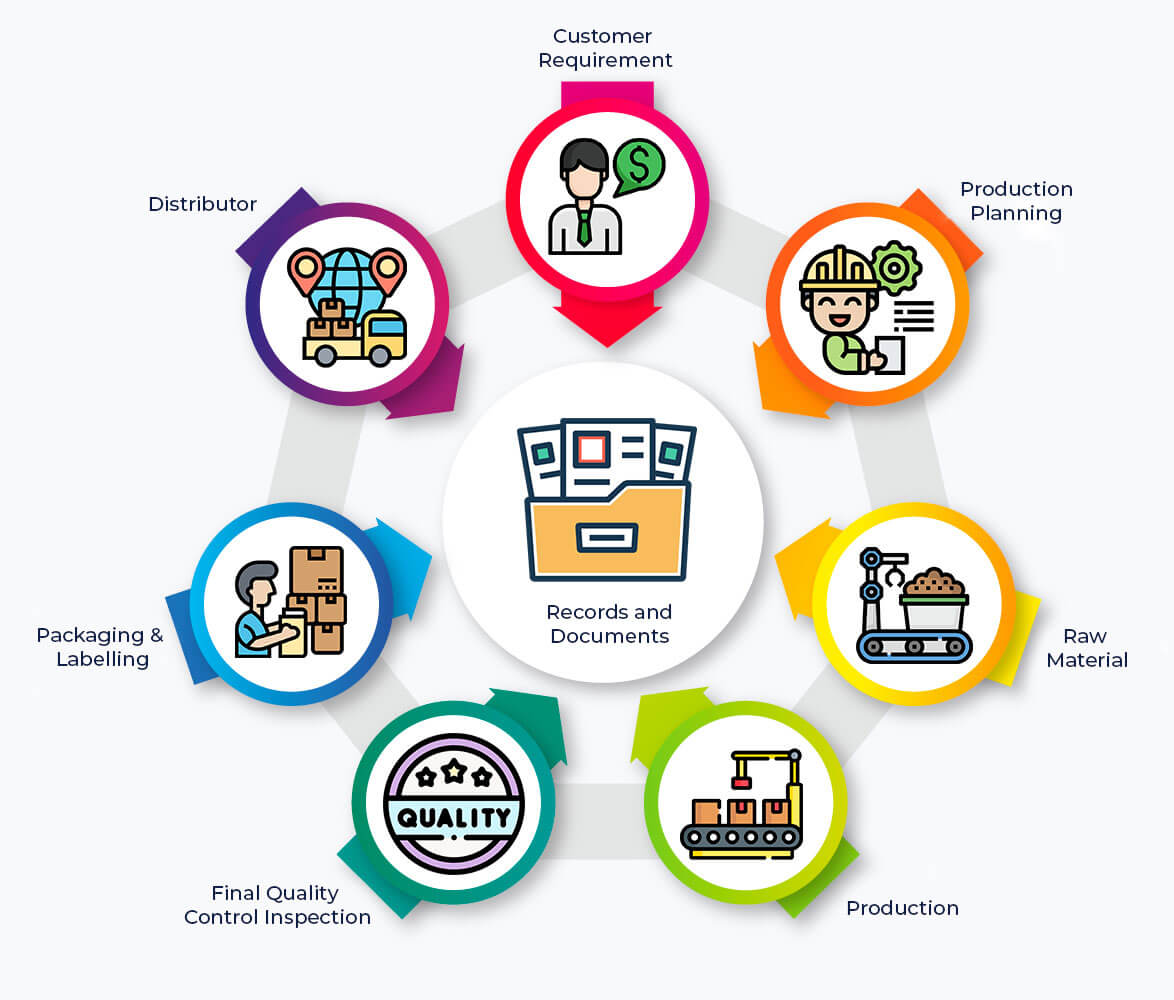
The term “Traceability” itself contains two words, ‘Trace’ and ‘ability’ which simply means it is an ability of your product to trace the history. It helps you to understand the origin of product and enables you to understand where the product come from and where it is delivered. Whenever we give identification to product, traceability is not always required but for a complete traceability, identification is an initial requirement. In medical device manufacturing, traceability can be maintained by using records and documents at each stage of medical device. It provides both tracks forward (Raw material to end user) and backward (End user to raw material). To get the information on each part and product, details should be recorded end to end from Customer requirement to final shipping to customer.
When any recall or non-conformity happens in a medical device industry, it is extremely important to find the origin or root cause of non-conformity. At that time, traceability plays main role to understand the root cause. It increases the response time to recalls and helps curtail the consecutive damage and improves manufacturing efficiency.
The traceability records with identification numbers from raw material to end user must include:
- 1. Customer/ sales orders
- 2. Production planning records
- 3. Raw material issue bills
- 4. Date of manufacturing
- 5. Quantity manufactured
- 6. In process inspection records
- 7. Quantity release for distribution
- 8. Final Inspection tests and reports
- 9. Packaging and labelling details
- 10. Distribution to end user details
Hence, it becomes crucial for an organization to prepare and implement a robust Quality Management System (QMS) to minimize manufacturing errors, maximize profits, save time, reduce inter-departmental discussions or disputes and give their customers a good user experience. The QMS implementation in itself has processes that ensure intra and inter-departmental coordination as a successful business requires that all departments are in sync with each other. The implementation of effective QMS is the key to any successful business and hence should never be compromised.
If you are looking for the right organization that can customize your Quality Management System (QMS) to your needs and help implement the same within your organization as if it was tailor-made for you, contact Maven Profcon Services LLP.