Changes After Brexit: What It Means for Your European Authorised Representative
Want to keep selling your medical devices in both the UK and the EU? After Brexit, the rules have changed for appointing authorised representatives.
If you’re a manufacturer outside the EU, you now need two separate authorised representatives—one in the UK and one in the EU. This is now required to continue placing your products in both markets.
For companies based in the UK selling medical devices in the EU, having a European Authorised Representative is now mandatory.
If you were using a UK-based representative earlier, you’ll need to switch to an EU Authorised Representative for medical devices to keep access to the EU market.
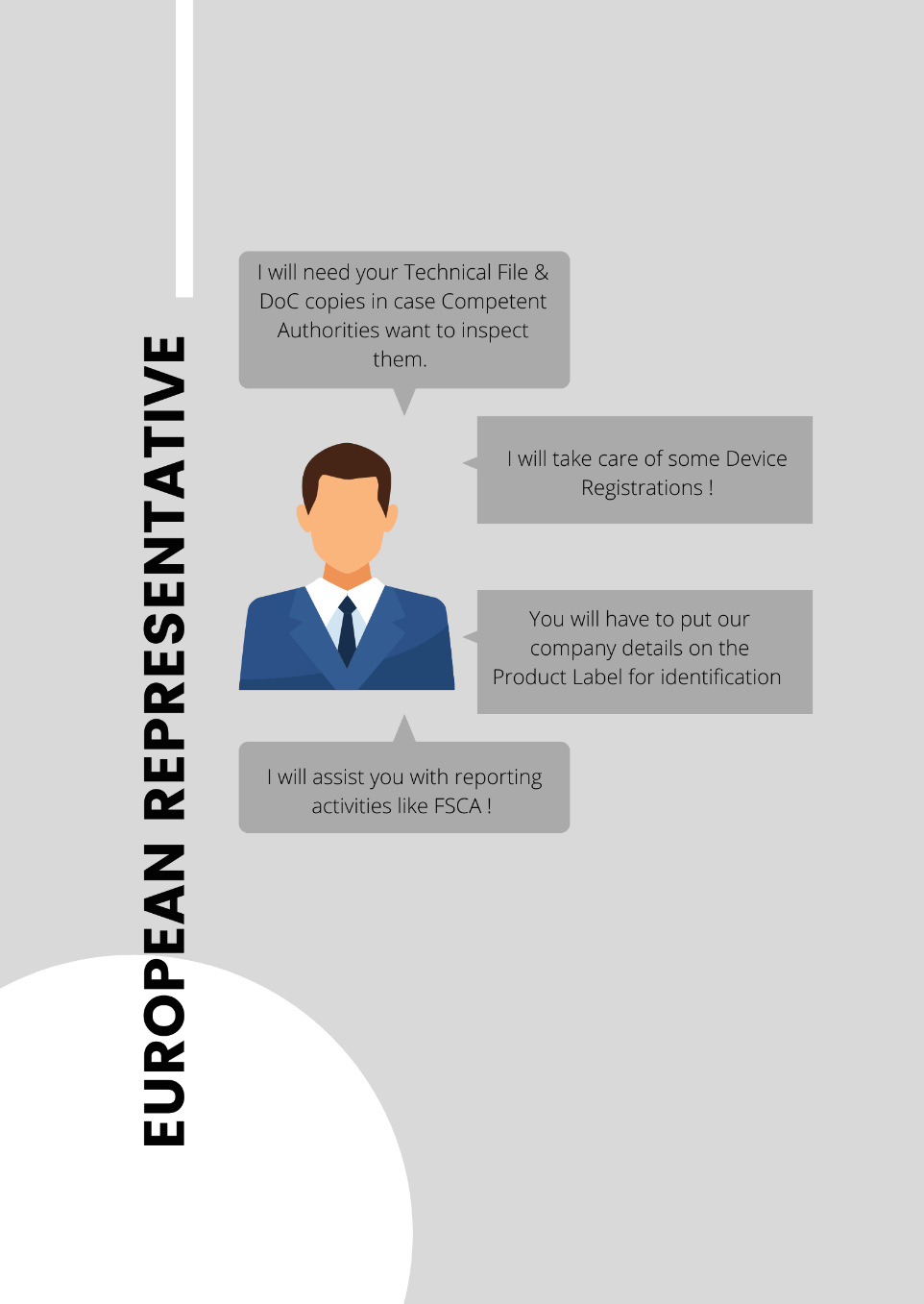
As a trusted medical device regulatory consultancy, we offer complete European Authorised Representative services to help you stay compliant and continue selling your products without interruption.
European Free Sales Certificate
Got your CE certificate and looking to expand your medical device sales globally? You might need a Free Sales Certificate to move forward with your distribution process.
This certificate is issued by the European Competent Authorities upon request from your European Authorised Representative. It’s an important document that allows you to freely sell or distribute your medical devices outside the EU.
Keep in mind, this certificate isn’t necessary for sales within the EU, as it only applies to markets outside the EU.
As a medical device regulatory consultancy, we offer a Free Sales Certificate through our European Authorised Representative to ensure your devices are ready for global distribution.