SaMD – Software as a Medical Device
If you are willing to certify your SaMD – Software as a Medical Device, Maven is all prepped to guide you!
So where did this term come from?
Well, nowadays, technology is making its way into everybody’s lives. Even the healthcare sector has not been left out. Software has become a part of many medical devices, and in some cases, software, on its own, qualifies as a medical device. Leading SaMD companies are continuously innovating to meet regulatory standards and improve patient outcomes.
What is Software as a Medical Device (SaMD)?
The term Software as a Medical Device (SaMD) is defined by the International Medical Device Regulators Forum (IMDRF) as “software intended to be used for one or more medical purposes that perform these purposes without being a part of a hardware medical device.”
Now, you might already know that as per EU MDR – 2017/745, there is a provision of a specific rule for SaMD medical devices. Additionally, regulatory authorities such as the FDA regulate Software as a Medical Device, ensuring compliance with strict safety and performance standards. If you’re looking for Software as a Medical Device companies that specialize in regulatory support, Maven is here to assist you.
For those navigating SaMD FDA approval processes, understanding the evolving compliance landscape is essential. Let Maven help you achieve regulatory success with your SaMD certification!
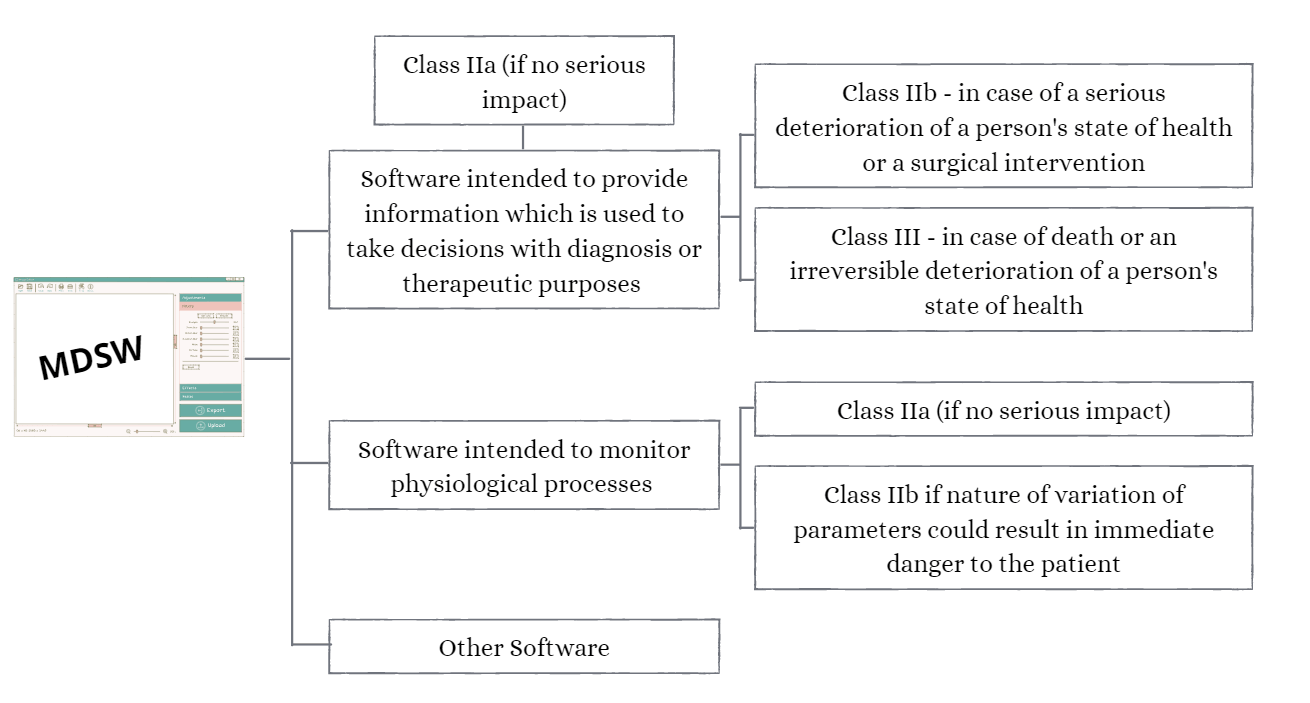
Rule No. 11: Software Classification Guidelines
Software intended to provide information which is used to take decisions with diagnosis or therapeutic purposes is classified as class IIa, except if such decisions have an impact that may cause:
- death or an irreversible deterioration of a person’s state of health, in which case it is in class III; or
- a serious deterioration of a person’s state of health or a surgical intervention, in which case it is classified as class IIb.
Software intended to monitor physiological processes is classified as class IIa, except if it is intended for monitoring of vital physiological parameters, where the nature of variations of those parameters is such that it could result in immediate danger to the patient, in which case it is classified as class IIb. All other software is classified as class I.
Software as a Medical Device vs. Software for Medical Devices: Key Differences
Below are the definitions provided in the MDCG guideline document – Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR” released in October 2019:
Software Driving or Influencing the Use of a Device
Software that is intended to drive or influence the use of a (hardware) medical device but does not perform a medical purpose on its own or generate medical information independently is categorized separately. It supports the operation or control of a medical device but does not fall under SaMD (Software as a Medical Device).
This type of software can:
- Operate, modify, or control the medical device through an interface (e.g., software, hardware) or via the device operator.
- Provide output related to the (hardware) functionality of that device.
Note: Software that drives or influences the use of a (hardware) medical device may qualify as an accessory for a medical device rather than standalone SaMD software.
Understanding Software as a Medical Device (SaMD)
Unlike software that merely supports hardware functionality, FDA Software as a Medical Device (SaMD) is a standalone software solution that serves a medical purpose independently. Medical device software companies develop SaMD solutions for diagnostics, treatment recommendations, and patient monitoring.
Common Software as a Medical Device examples include AI-driven diagnostic tools, mobile applications for chronic disease management, and software used for digital therapeutics.
By distinguishing between SaMD Software as a Medical Device and software that simply aids in hardware operations, regulatory compliance becomes clearer, ensuring patient safety and device effectiveness.
Examples of Software as a Medical Device (SaMD) and Device-Driven Software
Let us enlighten you with few examples of Software as a Medical Device – SaMD :
- Imaging Software to detect tumours
- Software that detects Myocardial Infarction
- information from trend reporting;
- Software used for EEG Analysis
- Software that receives measurements from transrectal ultrasound findings, age
Now let us take a look at a few examples of Software driving or influencing the use of a device –
- Software intended to measure & transmit blood glucose levels, calculate insulin dose required
- Melanoma image analysis software intended to drive a near – infrared laser light scanner
Also, Software may be qualified as MDSW regardless of its location (Like operating in cloud, on computer, on mobile phone, or an additional functionally on a hardware medical device)
- Software that is intended to operate a point of care test from a remote location
And, Software that is intended to be used by healthcare professionals or lay persons (e.g. patients or other users)
- Software that provides insulin dose recommendations to a patient regardless of the method of delivery of the prescribed dose, whether via an insulin pump, insulin pen or insulin syringe.
CE CERIFICATION FOR MEDICAL DEVICE SOFTWARE PROCESS
Now to get your CE Certificates for Medical Device Software, we have laid out the complete pathway of this regulatory process for you –
- 1st step would be to reveiw your Medical Device Software and classify it as per the Classification rules provided in EU MDR 2017/745
- Next step would be to establish QMS of the organization (Note – Class I Device – Not mandatory)
- Now let’s draft the Technical File as per the Annnex II and III of EU MDR
- Time to appoint the Authorized Representative and do not forget to get the agreement signed from them (As per Article 11 of EU MDR).
- Have you registered your company on EUDAMED Portal yet ? No? Go for it ! Do register your devices as well once the modules gets active !
- Class I MDSW? – Self declare the CE Mark otherr Class ? – get your TCF & QMS audited by the Notified Body
- Notified Body : Here is your CE Certificate ! Now you can affix CE mark on the Medical Device
MEDICAL DEVICE SOFTWARE – SaMD CLASSIFICATION AS PER EU MDR (2017/745) REGULATION
Classification of medical device software does not directly impact the patient but is primarily concerned with indirect harms resulting from failures.
To address these risks, the EU MDR (2017/745) Regulation introduced Rule 11, specifically for Software as a Medical Device (SaMD). This rule ensures proper risk management for active medical devices that provide critical information for patient care.
Additionally, regulatory bodies like the FDA have established guidelines for FDA Software as a Medical Device, ensuring compliance with safety and performance standards. Many SaMD companies actively develop innovative medical software while adhering to global regulations.
Allow us to explain that “Software is also defined as an Active Medical Device”, reinforcing its significance in modern healthcare solutions.
Annexure III of the MDCG 2019-11 guideline document has also provided the Risk Classification Framework for Medical Device Software based on Rule 11 of the EU MDR Regulation:
| State of Healthcare Situation / Patient Condition | Significance of Information Provided by the MDSW |
| High (Treat / Diagnose) | Medium (Drives Clinical Management) | Low (Informs Clinical Management) |
| Critical | Class III | Class IIb | Class IIa |
| Serious | Class IIb | Class IIa | Class IIa |
| Non-Serious | Class IIa | Class IIa | Class IIa |
HOW MAVEN CAN HELP WITH MDR SOFTWARE CE MARKING?
Why fear? Maven is here!
We have a specialized team of talented and trained technical experts take care of the requirements of the Medical Device Software Technical File Submission and the end-to-end CE marking process for your Medical Device Software (MDSW).
All the necessary actions related to the complete CE marking process like –
- Classifying your Medical Device Software as per the Classification Rules mentioned in the EU MDR (2017/745) regulation
- Already have the existing QMS? If yes, then updating the QMS as per the requirements available in the EU MDR regulation & If not, establishment of the complete QMS set
- Guidance on the technical documentation preparation including all necessary requirements like Software Risk Management, Software Validation, Post market activities, Clinical evaluation etc.
- Constant support from submission of technical file to Notified Body to responding to the queries raised by the Technical File Reviewer.
- Audit support for the initial audits as well as surveillance audit until the validity of the CE certificate to help you out with the queries raised by the auditor for smooth auditing process.