What is the CE Mark Logo?
A CE Mark logo is affixed on a product which implies that the manufacturer affirms that the product has been subjected to conformity assessment and complies with the requirements of the relevant product safety legislation of Europe.
- Scientific validity
- Analytical performance
- Clinical performance
Affixing the CE logo
Once the CE Logo is affixed, it denotes that the product is compliant with the European regulations and can be marketed throughout the European Economic Area. Therefore, affixing a CE logo is an essential step. For importers and exporters as well it is a critical step to affix the CE logo in order to sell products within the European Economic Area, which have been manufactured outside.
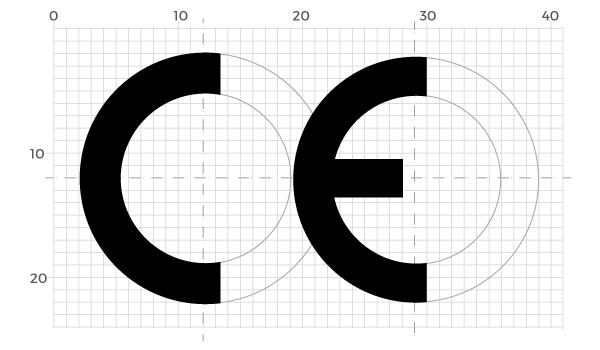
The CE Logo
Any changes in the CE Logo must preserve the proportions of the logo.
NOTE: The CE logo is formed by imperfect semi-circles. The arms on the main circle are one square longer at each end than ‘perfect’ and the central square bracket is a shorter one.
The CE mark must be at least 5 mm high. The CE mark must be placed on the product or its data plate. If it cannot be directly affixed on the product then it shall be placed on the product packaging and documents such as the user manual according to the regulatory requirements. The CE mark must be visible, legible and should be placed in a way that it cannot be removed asily.