Do you know what SOUP is? Unlike soup we eat which is made up of various known ingredients here in this SOUP the origin of ingredients is unknown. Let’s talk about Software of unknown province also known as “SOUP”
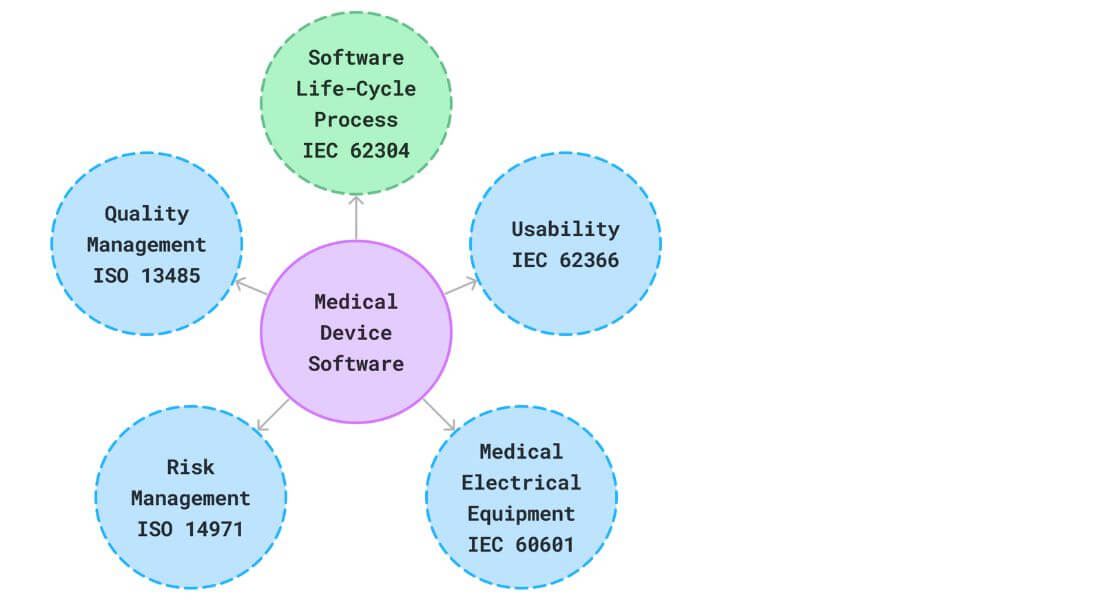
According to IEC 62304 software from unknown origin are those Software that is already created, widely accessible, and not specifically designed for integration into a medical device (referred to as “off-the-shelf software”, or software for which comprehensive development process records are unavailable)
What is Software of Unknown Provenance?
These are the software’s which are commercially available for various use or without any particular use and without tailored customization for public use. For such software development records are unknown as per requirement stated in IEC 62304 these kinds of software are known as software from unknown provenance. Unknown provenance can be explained as the whereabouts of software being unknown.
SOUP must be verified to guarantee it is safe and effective for use in healthcare applications, and it must adhere to the same regulatory standards as other software, such as bespoke software, according to the FDA. Testing the software and ensuring that it complies with regulatory requirements may be part of this process, along with examining the vendor’s software development methodology. Medical device manufacturers can cut costs and development time by using off-the-shelf (OTS) software, but doing so calls for cautious OTS software component management and selection to guarantee compliance with regulatory standards.
IEC 62304 Defines Software of Unknown Provenance (SOUP)
IEC 62304 defines Software of Unknown Provenance (SOUP) as software items used in a medical device but not developed according to the standard’s software lifecycle processes. This includes commercial off-the-shelf (COTS) software, open-source software, and legacy code. Since the development history and quality controls of SOUP are often unknown or unverifiable, IEC 62304 requires manufacturers to assess associated risks, apply risk control measures, and ensure the software’s suitability for its intended use. Proper documentation, validation, and justification for SOUP use are essential to ensure compliance and patient safety. Managing SOUP is critical to achieving regulatory approval for medical software devices.
Why is it necessary to manage and report the Software from unknown provenance?
Software with an unknown provenance also lacks the necessary IEC 62304 documents, such as the Design & Development file, testing, and validation document. As a result, in the event that a software malfunction that could endanger patients, the company is unable to track down the malfunction making it challenging to identify the malfunction.
The assessment of Software of Unknown Provenance (SOUP) extends beyond the initial release of the source code, as developers routinely generate updates for SOUP components. Some of these updates may address issues that can affect the functionality of your device software. Post-market surveillance plays a crucial role in the ongoing management and monitoring of SOUP.
Examples of SOUP in Medical Devices
Examples of SOUP in medical devices include operating systems like Windows or Linux, third-party libraries for image processing, open-source encryption tools, database engines such as MySQL, and commercial communication protocols. These components are often integrated without full development documentation, requiring thorough risk assessment and validation to ensure device safety and compliance.
Off-the-Shelf (OTS) Software vs SOUP
| Aspect | Off-the-Shelf (OTS) Software | Software of Unknown Provenance (SOUP) |
| Definition | Commercial software available for general use | Software with unknown or unverifiable development process |
| Documentation | Usually well-documented | Often lacks complete documentation |
| Development Control | May follow known quality standards | Development process is not fully known |
| Risk Management | Required for medical use | Critical due to unknown origins |
| Usage in Medical Devices | Common with validation | Allowed with strong justification and risk controls |
Managing SOUP with a Software Bill of Materials (SBOM)
Managing SOUP with a Software Bill of Materials (SBOM) enhances transparency, security, and regulatory compliance in medical device software. An SBOM is a comprehensive inventory of all software components, including SOUP, used in a device. It identifies each component’s origin, version, and dependencies, allowing manufacturers to track vulnerabilities, apply patches, and assess risks effectively. SBOMs support proactive risk management, facilitate audits, and streamline regulatory submissions. By documenting SOUP within an SBOM, organizations ensure traceability, reduce the likelihood of unapproved changes, and demonstrate due diligence. This approach aligns with cybersecurity guidelines and strengthens the safety and reliability of medical software systems.
Navigating Compliance with IEC 62304 for SOUP
Navigating compliance with IEC 62304 for Software of Unknown Provenance (SOUP) requires a structured risk-based approach. Since SOUP lacks a verifiable development history, manufacturers must identify all SOUP components, assess their intended use, and analyze potential hazards. Risk control measures, such as isolation, monitoring, or redundancy, must be implemented as needed. Thorough documentation, including justification for use, risk assessments, and validation evidence, is essential. Software safety classification determines the level of scrutiny. Regular updates, vulnerability tracking, and integration testing are also required. Complying with IEC 62304 ensures that SOUP does not compromise medical device performance, patient safety, or regulatory approval.
Management of SOUP in medical devices in future
The trajectory of Software of Unknown Provenance (SOUP) management in medical settings is poised for significant advancements. As technology evolves, the management of SOUP in healthcare environments is set to undergo transformative changes. These developments are likely to be characterized by more sophisticated strategies, enhanced tools, and refined processes to ensure the effective integration and oversight of SOUP in medical applications. Staying ahead in SOUP management is not only a necessity for regulatory compliance but also a crucial element in optimizing the efficiency and reliability of medical systems. The evolving landscape promises a future where healthcare professionals can harness the benefits of SOUP while maintaining a robust and adaptable framework for its seamless integration.
The future of managing Software of Unknown Provenance (SOUP) in medical settings is poised for substantial advancements driven by technological evolution. Anticipated developments include sophisticated strategies, enhanced tools, and refined processes to seamlessly integrate and oversee SOUP in medical applications. Staying ahead in SOUP management is crucial not only for regulatory compliance but also for optimizing the efficiency and reliability of medical systems. The evolving landscape foresees a future where healthcare professionals can leverage the benefits of SOUP within a robust and adaptable framework.