Registration process
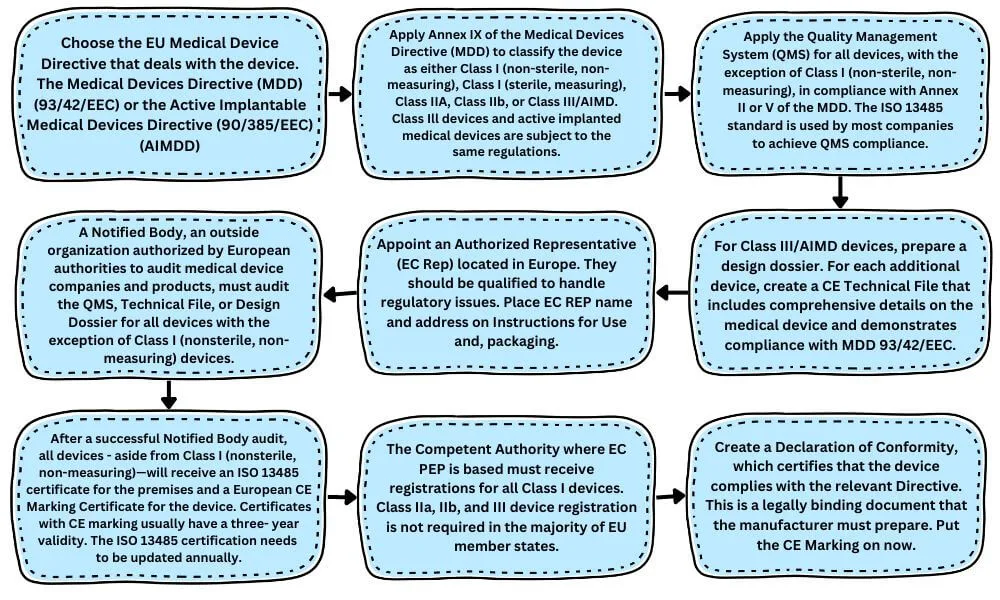
Europe Approval Process for Medical Devices
Europe (CE) Medical Device Approval Process: A CE Mark certificate is required for the commercialization of medical and IVD products inside the European Union. A device’s compliance with the Medical Devices Directive (MDD), In Vitro Diagnostic Device Directive (IVDD), or Active Implantable Medical Device Directive (AIMD) as they relate to the product is confirmed by this certification.
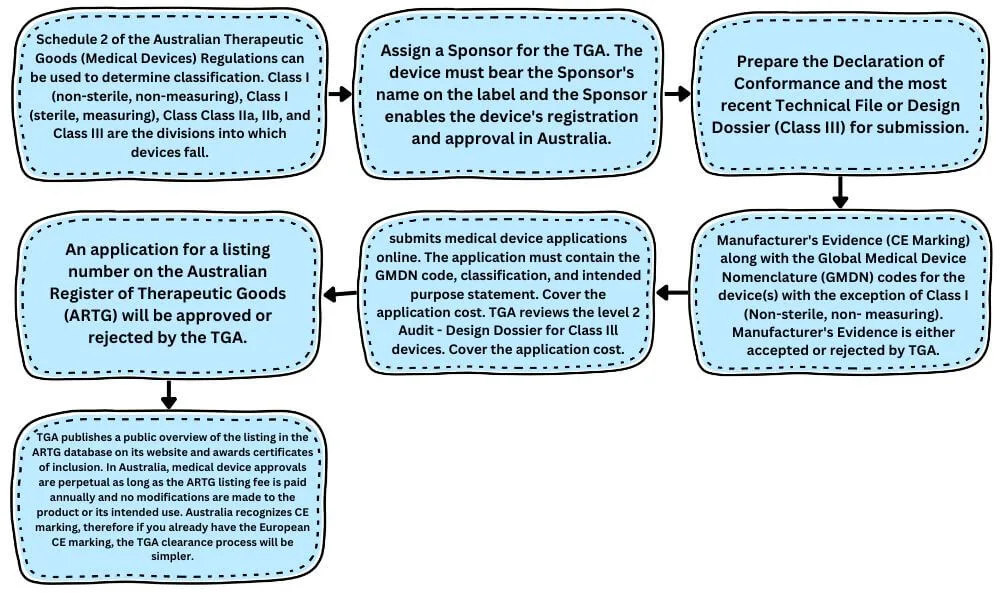
The Australian TGA Approval Process for Medical Devices
Manufacturers of IVDs and medical devices must list their products in the Therapeutic Goods Administration (TGA)-regulated Australian Register of Therapeutic Goods (ARTG) in order to sell their products in Australia.
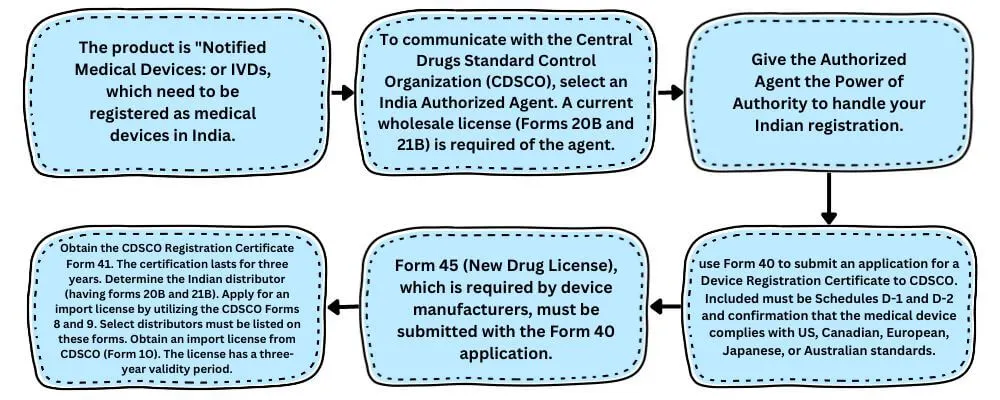
India Approval Process for Medical Devices
The Drugs and Cosmetics Rules 44 govern the regulations surrounding the registration of these items. Enumeration of medical devices: Blood Component Bags, Blood Grouping Sera, Bone Cements, Cardiac Stents, Catheters, Condoms, Drug Eluting Stents, Disposable Hypodermic Needles, Disposable Hypodermic Syringes, Disposable Perfusion Sets, Internal Prosthetic Replacements, Intra Ocular Lenses, Intra Uterine Devices, IVD Devices for HIV, HBsAG, and HCV, Orthopedic Implants, Scalp Vein Sets, Skin Ligatures, Surgical Dressings, Sutures and Staplers, Tubal Rings, Umbilical Tapes.
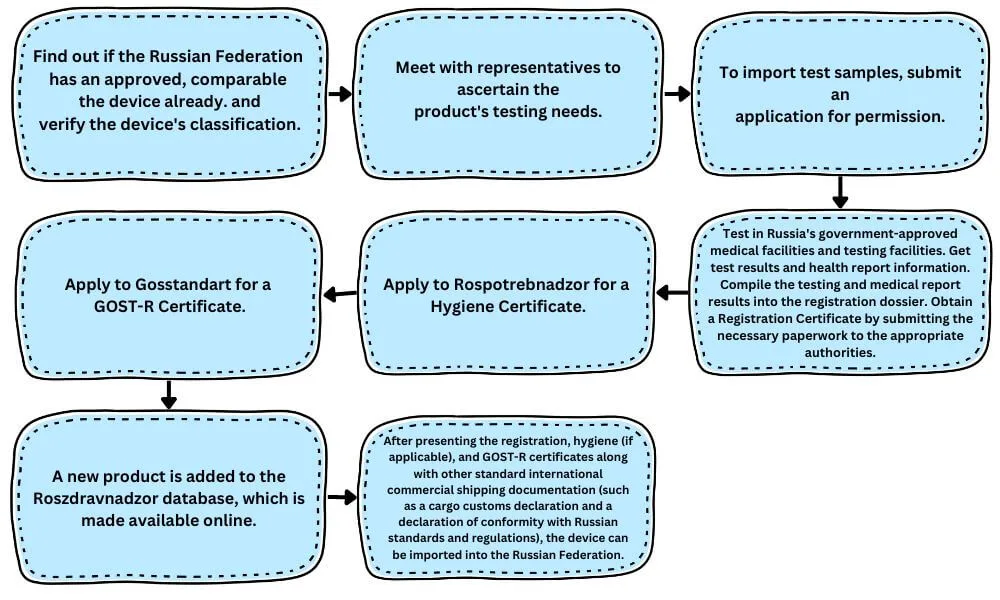
Russian medical device registration procedure
It is imperative to collaborate with a local specialist who is knowledgeable about the process and can guide the device through to a successful registration, as the Russian medical device registration procedure is intricate and there are limited English-language papers available. An overview of the sequence in which specific tasks are completed during the registration procedure.