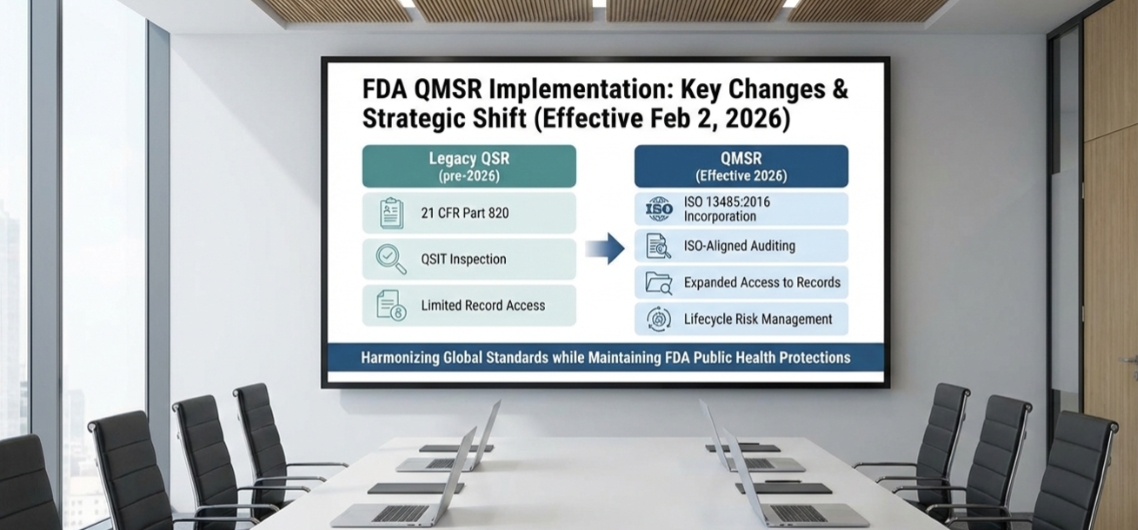
The U.S. Food and Drug Administration (FDA) has finalized a regulatory modernization with the introduction of the Quality Management System Regulation (QMSR), which will amend the long-standing Quality System Regulation (QSR) under 21 CFR Part 820. Effective February 2, 2026, QMSR represents a strategic shift toward global harmonization by incorporating ISO 13485:2016 by reference, while retaining FDA-specific regulatory expectations necessary to protect public health.
This transition is not merely a structural or terminological update. It fundamentally reshapes how quality systems are organized, inspected, and enforced for medical device manufacturers marketing products in the United States.
Transition to ISO-Aligned Terminology and Documentation
One of the most visible changes under QMSR is the shift away from traditional FDA-specific terms such as Device Master Record (DMR), Design History File (DHF), and Device History Record (DHR). In their place, manufacturers will increasingly rely on ISO-aligned concepts such as the Medical Device File and Design & Development File, consistent with ISO 13485 and ISO 9000 vocabulary.
This alignment supports FDA’s long-standing goal of international harmonization, enabling manufacturers with global operations to maintain a more unified documentation structure while still meeting U.S. statutory requirements.
Expanded FDA Access to Quality System Records
Under the previous QSR framework, certain quality system records—most notably management review outputs and internal audit reports—were explicitly exempt from FDA inspection. These exemptions are not carried forward under QMSR. As a result, FDA investigators may now review management review minutes, internal audit reports, supplier audit records, training documentation, and audit checklists during inspections, when requested.
This change significantly increases expectations for transparency, objectivity, and documentation rigor across the entire Quality Management System. Manufacturers should assume that internal quality records must be inspection-ready at all times.
FDA-Specific Requirements Remain Fully Enforceable
Although QMSR adopts ISO 13485 as its foundation, it does not dilute or replace existing FDA-specific regulatory obligations. Medical Device Reporting (MDR), labeling controls, and Unique Device Identification (UDI) requirements continue unchanged. While ISO 13485 addresses these topics at a high level, FDA will continue to enforce its detailed and prescriptive requirements to ensure traceability, post-market vigilance, and regulatory compliance.
This dual structure—ISO alignment combined with U.S.-specific enforcement—ensures there are no regulatory gaps between international standards and FDA statutory mandates.
Risk-Based Quality Management Across the Product Lifecycle
Risk management under QMSR is no longer limited to design and development activities. Manufacturers are now expected to integrate risk-based thinking throughout the entire Quality Management System, including supplier controls, manufacturing processes, post-market activities, and functionality-based failure scenarios.
This broader approach requires organizations to identify, assess, and control risks related not only to product design, but also to process performance, supplier reliability, and potential use-related or functional failures. FDA’s enforcement of this requirement will be closely tied to its core mission of ensuring device safety and effectiveness.
New Inspection Paradigm and CAPA Expectations
The FDA is retiring the Quality System Inspection Technique (QSIT) and moving toward an inspection approach aligned with ISO-style process auditing. This shift places greater emphasis on system effectiveness, process interactions, and continuous improvement rather than checklist-based inspections.
In parallel, Corrective and Preventive Action (CAPA) practices must comply with ISO 13485 requirements as incorporated into QMSR, while also meeting FDA expectations for investigation depth, root cause analysis, effectiveness verification, and risk-based decision-making. CAPA under QMSR is not evaluated solely through an ISO lens, but through FDA’s regulatory perspective focused on patient safety and product performance.
Combination Products and 21 CFR Part 4 (Regulation of Combination Products) Alignment
As part of the QMSR final rule, FDA has made conforming amendments to 21 CFR Part 4 to ensure continued alignment of combination product CGMP requirements with the new ISO 13485–based Quality Management System Regulation. These amendments do not introduce new CGMP obligations for combination product manufacturers. Instead, they update references from the legacy QSR to QMSR and clarify that device-related quality system requirements within Part 4 will now be evaluated against the QMSR framework. Existing streamlined and full CGMP compliance approaches under Part 4 remain unchanged.
Reference:
Medical Devices; Quality System Regulation Amendments